RUFY4 deletion prevents pathological bone loss by blocking endo-lysosomal trafficking of osteoclasts
Published in Cell & Molecular Biology

Healthy bone is maintained by a dynamic balance between osteoblasts, which synthesize new bone matrix, and osteoclasts, which dissolve old or impaired bone tissue. However, if this process becomes imbalanced due to excessive osteoclast activity, bone-loss diseases like osteoporosis can occur1,2. Treatments for these diseases are primarily based on either promoting the function of osteoblasts or blocking the function of osteoclasts, but for long-term treatment, the communication between these two cells must kept intact to avoid side effects3,4,5.
Upon contact with the bone surface, osteoclasts reorganize their cytoskeleton to form 'sealing zones', actin-rich ring-like structures, for cell-matrix adhesion. Then, they secrete acid hydrolases like cathepsin K (CTSK) from lysosomal vesicles through a ruffled border, a unique membrane area facing the side of the sealing zones, to break down bone6. The biogenesis, trafficking, and exocytosis of these lysosomes are therefore critical for proper bone resorption. However, the precise mechanisms by which lysosomes are regulated in osteoclasts remain poorly understood.
In this study (https://doi.org/10.1038/s41413-024-00326-8), we identified a gene, RUN and FYVE domain-containing protein 4 (RUFY4), which was upregulated by RANKL, a cytokine that activates osteoclast differentiation and function, through GeneChip analysis. This discovery led us to create whole-body Rufy4 knockout mice and explore the function of RUFY4 in osteoclasts. Studies including micro-CT and histological analyses showed that Rufy4-deficient mice had increased trabecular bone mass with no change in osteoblast and osteoclast numbers. Notably, these mice showed no difference in bone formation indicators, but only reduced osteoclast activity, as evidenced by shorter contact lengths with bone and shallower bone resorption pits. Next, we sought to determine the role of RUFY4 at the cellular level. Consistent with our in vivo results, Rufy4 deficiency did not affect osteoblast and osteoclast differentiation. However, we found that osteoclast function was markedly blocked in the absence of RUFY4 using resorption pit formation assay with hematoxylin or wheat germ agglutinin (WGA), c-telopeptide of type Ⅰ collagen (CTX-1) ELISA assay, calcium colorimetric assay, and CTSK secretion assay.
Given that RUFY4 interacts with Rab77, which plays a key role in endo-lysosomal transport8, we investigated whether the impaired bone resorption of Rufy4-/- osteoclasts is due to endo-lysosomal trafficking dysfunction. Experiments showed that the mean fluorescence intensity of Rab7 and LAMP2, common late endosome and lysosome markers, was significantly reduced within actin rings in the absence of RUFY4. Acidic lysosome maturation and lysosomal intracellular activity were also reduced in Rufy4-/- osteoclasts. As a result of impaired lysosomal trafficking, Rufy4-/- osteoclasts lacked ruffled borders. Mechanistic studies using co-immunoprecipitation and immunofluorescence analyses confirmed that RUFY4 forms a bridge in endosome-lysosome fusion.
Finally, we explored the potential role of RUFY4 in bone diseases. To do so, we used animal models of inflammatory bone-loss disease and postmenopausal osteoporosis. In both models, Rufy4 knockout mice were significantly protected from osteoclast-mediated bone loss.
In summary, knocking out Rufy4 in mice only reduced osteoclast resorption activity without affecting osteoclast and osteoblast differentiation, resulting in heavier trabecular bone mass. Mechanistically, RUFY4 acts as an adaptor protein between Rab7 on late endosomes and LAMP2 on lysosomes, promoting endo-lysosomal trafficking, maturation, CTSK secretion, and ultimately bone resorption. This study represents RUFY4 as a novel and potential target to treat bone-loss diseases like osteoporosis.
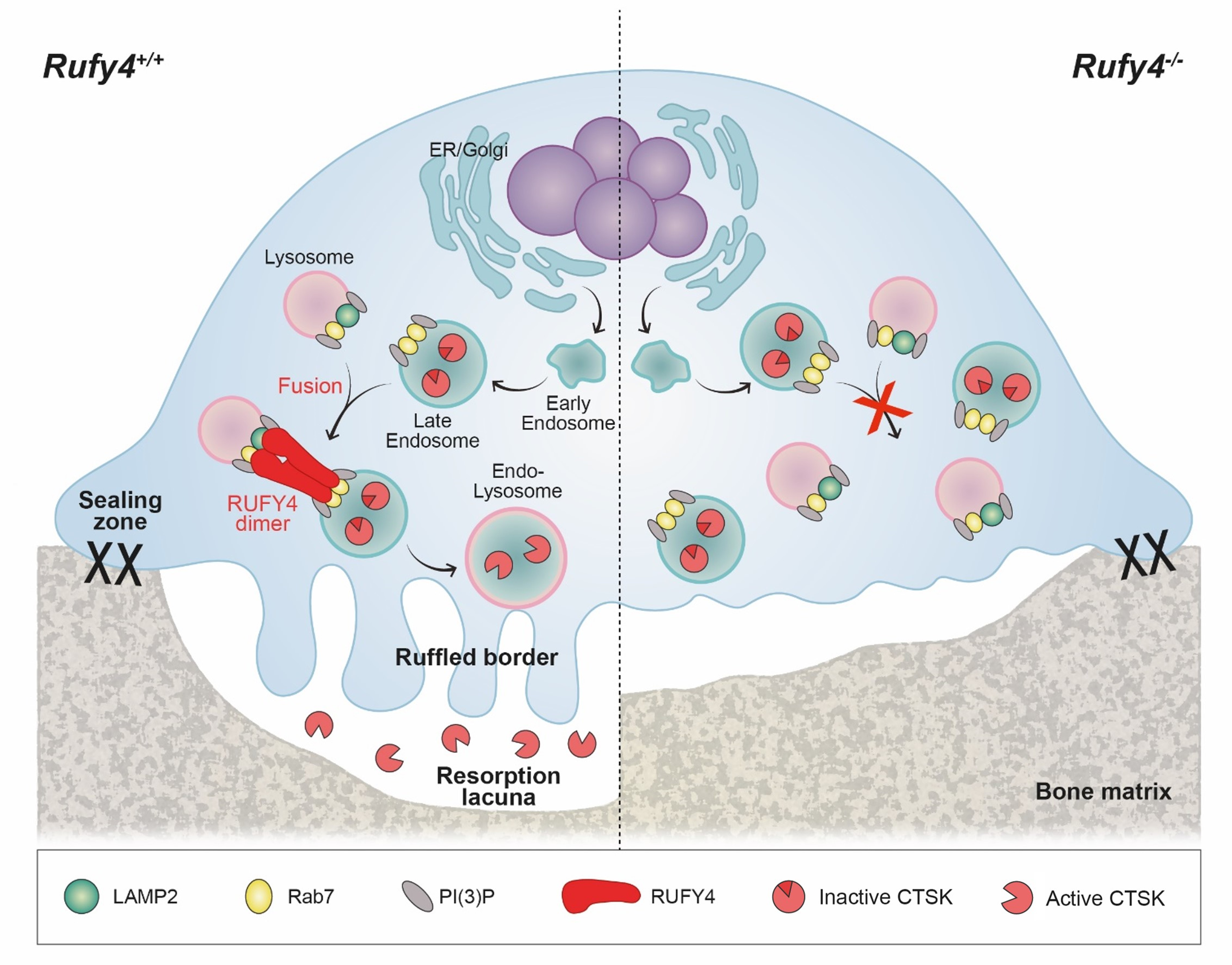
A schematic illustration of the mechanism by which RUFY4-induced endo-lysosomal trafficking promotes bone resorption in osteoclasts. The RUFY4 dimer promotes the fusion of late endosomes and lysosomes by interacting with Rab7 and LAMP2. The endo-lysosomes then move to the ruffled border and undergo exocytosis. The secretion of lysosomal hydrolases, including active CTSK, into the extracellular space near the bone leads to the emergence of resorption lacunae.
Dr. Soo Young Lee is the corresponding author of this paper, with Ph.D. student Minhee Kim and Dr. Jin Hee Park serving as co-first authors.
References
- Zaidi M. Skeletal remodeling in health and disease. Nat Med 13, 791-801 (2007).
- Delaisse JM, Andersen TL, Kristensen HB, Jensen PR, Andreasen CM, Soe K. Re-thinking the bone remodeling cycle mechanism and the origin of bone loss. Bone 141, 115628 (2020).
- Tu KN, et al. Osteoporosis: A Review of Treatment Options. P T 43, 92-104 (2018).
- Khosla S, Hopbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endo 5, 898-907 (2017).
- Cao X. Targeting osteoclast-osteoblast communication. Nat Med 17, 1344-1346 (2011).
- Feng X, Teitelbaum SL. Osteoclasts: New Insights. Bone Res 1, 11-26 (2013).
- Terawaki S, et al. RUN and FYVE domain-containing protein 4 enhances autophagy and lysosome tethering in response to Interleukin-4. J Cell Biol 210, 1133-1152 (2015).
- Wang TL, Ming Z, Wu XC, Hong WJ. Rab7: Role of its protein interaction cascades in endo-lysosomal traffic. Cellular Signalling 23, 516-521 (2011).
Follow the Topic
-
Bone Research

This journal highlights the breakthrough discoveries in basic and clinical aspects of bone biology, pathophysiology and regeneration, as well as other significant findings related to bone.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in