SARS-CoV-2 fragments and immune echoes in the bloodstream: who is leading the dance?
Published in Microbiology, Biomedical Research, and Immunology
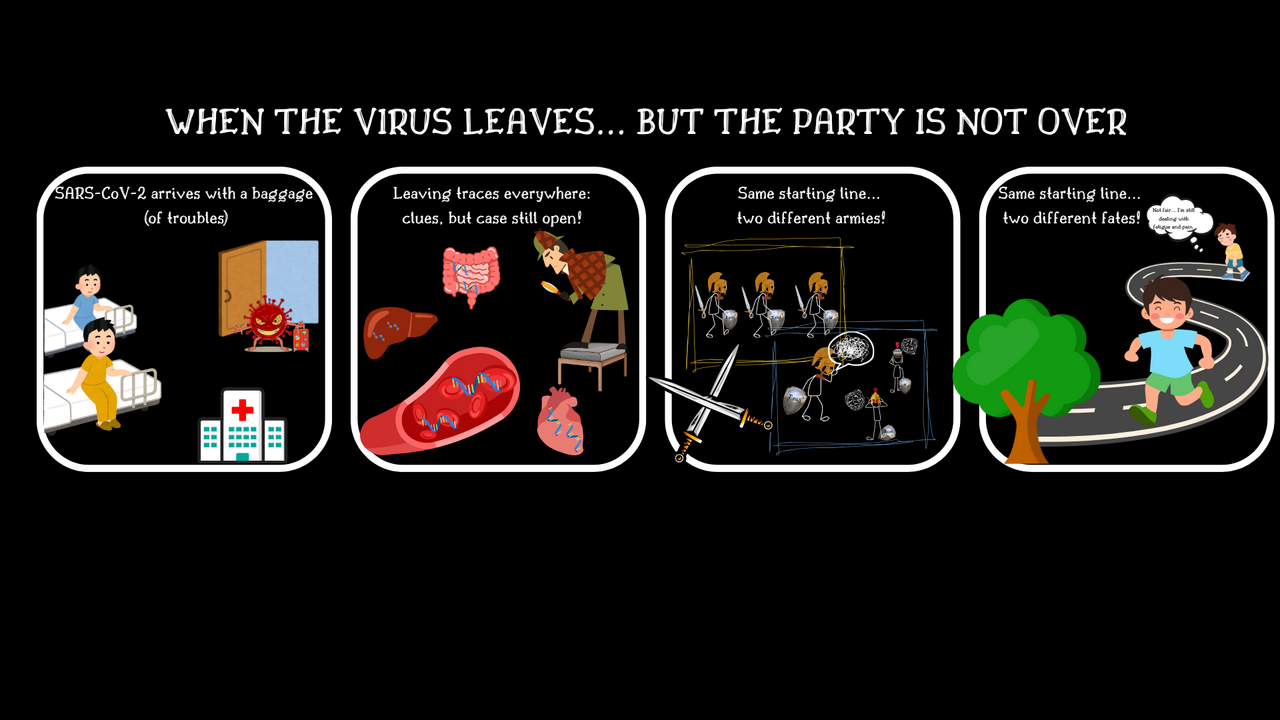
After SARS-CoV-2 virus is eliminated from the respiratory tract, many individuals continue experiencing signs and symptoms such as fatigue, shortness of breath, and cognitive issues, which negatively impact their daily activities. This condition is called long-COVID, and many underlying hypotheses have emerged so far, including immune dysfunction and viral persistence. Although SARS-CoV-2 is a virus with a respiratory tropism, viral traces - such as proteins or genome fragments - have been found in the blood as well as in other organs, suggesting a complex pathogenesis with a potential systemic component.
This question—why do some people continue to feel unwell long after recovering from the virus?—is what sparked our study.
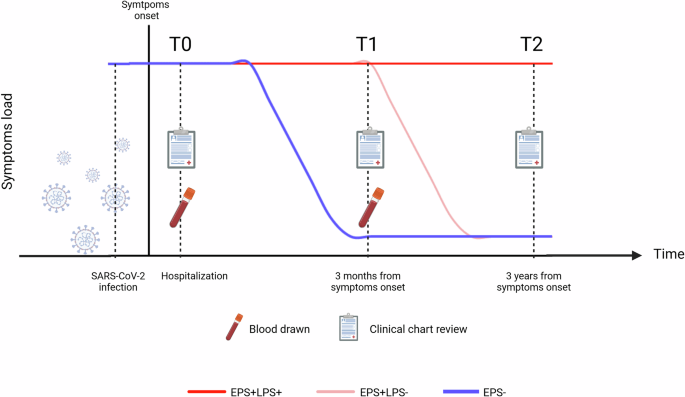
Working within the EuCare Study Group, an international research consortium, we followed a group of hospitalized, unvaccinated individuals from the first wave of the pandemic. We analyzed their immune responses at two timepoints: during the acute infection and three months later. Additionally, when symptoms persisted, we evaluated the association between presence of persistent symptoms at three years after infection with earlier immune dysregulation. Our focus: interplay between viral fragments and immune dysregulation in the development of persistent symptoms after SARS-CoV-2 infection.
Interestingly, while persistent circulating viral fragments were detected in almost all individuals – regardless of whether they had persistent symptoms - and while inflammation was widespread, the immune responses of subjects with persistent symptoms didn’t follow the expected choreography. Indeed, T cells, B cells and antibodies specifically designed for SARS-CoV-2 failed to mature properly, and even other T cells, non-specifically designed for the virus, became misdirected, drifting toward terminal differentiation. Most intriguingly, we found a link between higher levels of viral RNA in the blood and increased antibody-dependant cellular cytotoxicity (ADCC), BUT only in individuals with persistent symptoms. ADCC reflects the immune system's attempt to destroy infected cells using antibodies. This raises the questions: could the presence of viral fragments indicate ongoing, localized infection only in those with persistent symptoms? Is this misdirected immune response a reaction to something still hiding in the body? Are there other unknown factors altering such immune responses?
Why it matters?
In the backdrop of a silent and persistent burden, that is long-COVID, our work adds another piece to the puzzle—helping researchers, clinicians, and patients understanding that persistent symptoms are not “in their head,” but rooted in the biology of the immune system.
Beyond COVID-19, this journey has taught us a broader lesson: future pandemics are inevitable. Globalization, urbanization, and climate change make them more likely—and perhaps more frequent. COVID-19 exposed critical gaps in our preparedness—not just in emergency response, but in understanding and managing long-term consequences. To be ready for the next pandemic, we need to understand the one we’re still living with. That means deciphering how the virus and immune system interact—not just in the acute phase, but in the months and years that follow.
We hope our findings will fuel further research—and, ultimately, better treatments.
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
What a fascinating read! Thank you for sharing.