.png)
Humans have been battling viruses since the beginning of time. These microscopic entities not only cause acute discomfort but can lead to long-term health problems in the host. Influenza A virus (IAV or flu) is a well-known seasonal virus that, while generally manageable in healthy individuals, can cause serious complications in vulnerable groups, such as pregnant women. Pregnant women infected with IAV face increased risk of pregnancy complications like spontaneous abortion, lower birth weight, and stillbirth [1]. Notably, recent epidemiological studies suggest that prenatal IAV infection is also associated with a higher incidence of neurodevelopmental disorders (NDDs) in offspring [2]. However, the specific mechanisms by which exposure to IAV during pregnancy may lead to offspring NDDs remain unclear.
In the late 1990s and early 2000s, Fatemi and colleagues pioneered the use of mouse-adapted IAV to investigate the effects of IAV infection during pregnancy on fetal brain development in mice. Their research revealed that a neurotropic strain of IAV led to altered neonatal brain development, including reduced cortical thickness, dysregulated corticogenesis, and neuropathology resembling that observed in patients with NDDs [3-4]. However, the popularity of using live viruses in such studies declined once it was determined that the mother’s immune response to the virus—rather than the virus itself—was leading to these developmental abnormalities [5]. Since then, immunostimulants like viral mimic poly I:C have become the preferred tools for modeling maternal immune activation (MIA) in rodents. While these studies have deepened our understanding of MIA mechanisms, they do not fully capture the complexities of live viral-induced immune responses. Therefore, we aimed to use a non-neurotropic strain of mouse-adapted IAV (which replicates the seasonal flu in humans) to test two leading hypotheses in the MIA field: one - that maternal adaptive immune cells called T helper 17 lymphocytes are critically involved in orchestrating inflammatory cascades that signal to the fetus [6]; and two - that fetal brain cortical patterning is disrupted and that this disruption may involve resident brain immune cells [7].
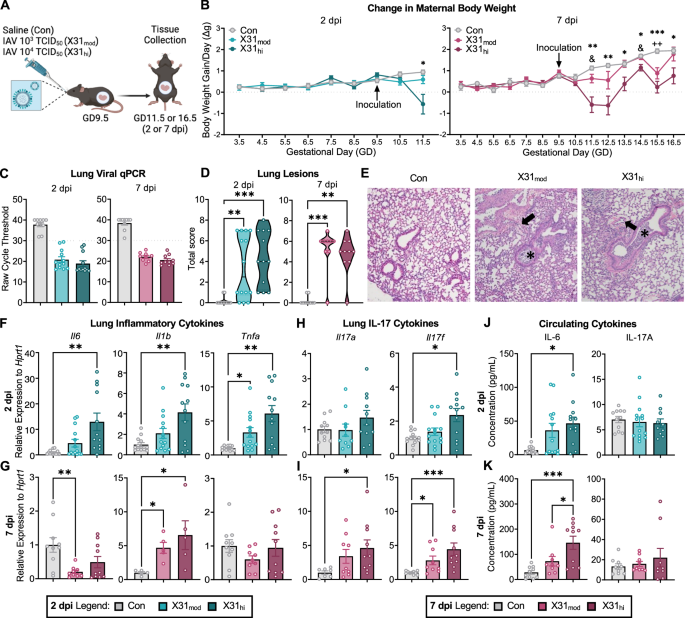
The corresponding author, Dr. Antonson, has previously shown that a moderate infectious dose of IAV in pregnant mice did not lead to fetal brain abnormalities [8]. Therefore, we decided to add a dose ten times higher to determine if an infection severity threshold exists for the onset of aberrant fetal brain development. Dr. Antonson’s previous work also focused on the peak adaptive immune response—approximately seven days post inoculation (dpi). I was interested in re-examining this time point (7 dpi) as well as 2 dpi, which approximates peak innate anti-viral immunity and corresponds to the timeframe within which immunostimulant-induced immune responses occur.
As expected, we found that intranasal inoculation with live IAV led to suppressed maternal body weight gain throughout gestation, which was more severe in the high-dose IAV group. We were particularly interested in what inflammatory signals were upregulated in maternal circulation, as these molecules have the potential to cross and/or negatively impact the maternal-fetal interface (i.e., the placenta). We observed an increase in circulating maternal pro-inflammatory cytokine interleukin (IL)-6 at both time points in a dose-dependent manner. This aligned with previous findings in the field, as IL-6 has been implicated in disrupted fetal development since the onset of MIA modeling [9]. Interestingly, we did not see any changes in circulating maternal IL-17, which is a cytokine that has garnered attention in recent years due to studies demonstrating that it and its primary parent cell, T helper (TH)-17, are necessary and sufficient to induce fetal neocortical abnormalities in poly I:C-MIA models.
We then turned to the intestines, which are the largest source of IL-17 producing TH17 cells. Here, TH17 cells take on a variety of phenotypes to either provide protection against harmful bacteria or confer tolerance to commensal bacteria. Single-cell analysis via flow cytometry revealed the opposite of our first hypothesis: a higher IAV dose led to decreased maternal intestinal IL-17 and TH17 cells at both 2 and 7 dpi.
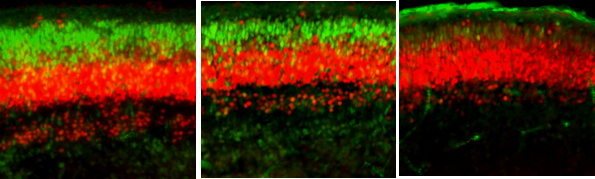
At this point, I was convinced that we would not see changes in the fetal cortex as a sufficient portion of MIA literature suggests that maternal intestinal IL-17 is necessary for the manifestation of neocortical malformations [10]. However, I proceeded with quantifying cortical neuronal layers at 7 dpi, or embryonic day (E)16.5 (these layers are not yet distinct at E11.5, or 2 dpi). Much to my surprise, I saw a reduction in upper excitatory neurons in layer II/III (characterized by reduced SATB2 fluorescence and cell count) and reduced cortical thickness overall, which is consistent with immunostimulant-MIA literature. Interestingly, this reduction was only evident in our high dose IAV group. Bulk RNA-sequencing of the whole fetal brain revealed downregulated gene ontology terms associated with cortical lamination and neurodevelopment, further supporting these findings. Candidate genes for NDDs, as defined by the Developmental Brain Disorder Gene Database (DBD) and the Simons Foundation Autism Research Initiative (SFARI), were also differentially expressed in fetal brains from high-dose IAV dams compared to control.
We then wanted to explore potential cellular mediators involved in this disrupted neocortical development. Microglia, the brain’s resident immune cells, are among the first cells to migrate to the embryonic brain, where they aid in early processes such as neocortical development. Knowing that the most commonly used microglia/macrophage marker, ionized calcium-binding adapter molecule 1 (Iba1), would identify microglia and border associated macrophages (BAMs)—both of which are present in the embryonic brain—I co-stained with macrophage-specific marker CD206. As it goes in science, the cell I merely meant to exclude at first (BAMs in this case) ended up delivering the most interesting findings. While microglia count remained unchanged, I observed an increase in BAMs in high-dose IAV fetal brains at E16.5, specifically within the meninges, which border the outer cortex. This was quite exciting as few groups have explored embryonic BAMs, especially in the context of MIA (one group had described MIA-induced accumulation of BAMs in the embryonic choroid plexus [11]. Intriguingly, choroid plexus BAM numbers were not impacted in our model). We then investigated functional differences in these brain macrophages and found elevated expression in phagocytic lysosome marker CD68 across all Iba1+ cells. Thus far, it appears that our second hypothesis is proving to be true. Our ongoing research is now exploring whether and how meningeal BAMs and parenchymal microglia may contribute to aberrant neocortical development following maternal IAV infection.
Overall, our manuscript demonstrates that certain features of MIA are conserved between immunostimulant and live virus models, while others are not, which highlights the importance of using biologically relevant models to close the gap between human epidemiological studies and preclinical mechanistic studies. Furthermore, our work supports the existence of an infection severity threshold whereby mom must be exposed to a high viral titer, or experience more severe disease, during her pregnancy before fetal brain abnormalities are likely to be observed.
References
- Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012; 207(3 Suppl): S3-S8.
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch Gen Psychiatry. 2004 Aug 1;61(8):774.
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–9.
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999; 4(2):145-154.
- Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int j dev neurosci. 2005 Apr;23(2–3):299–305.
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–9.
- Reemst K, Noctor SC, Lucassen PJ, Hol EM. The indispensable roles of microglia and astrocytes during brain development. Front Hum Neurosci. 2016.
- Antonson AM, Kenney AD, Chen HJ, Corps KN, Yount JS, Gur TL. Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain. Brain Behav Immun. 2021;96:28–39.
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702.
- Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549:528–32.
- Cui J, Shipley FB, Shannon ML, Alturkistani O, Dani N, Webb MD, et al. Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Dev Cell. 2020;55:617–628.e6.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in