Shifting the paradigm for non-contact anterior cruciate ligament rupture to emphasize intrinsic genetic risk in humans and dogs
Published in Healthcare & Nursing, Genetics & Genomics, and Biomedical Research
Why Study ACL Rupture in Both Dogs and Humans?
ACL rupture represents a significant burden on health systems and animal care (1). In humans, the condition often leads to long-term disability, secondary injuries, and early-onset osteoarthritis (2,3). Similarly, in dogs, it impairs mobility, diminishes quality of life, and increases the economic and emotional strain on owners (1). Studying canine ACL rupture provides a unique opportunity. Dogs exhibit breed-specific risk profiles and strong genetic predispositions to disease, making them an exceptional translational model (4,5). Their relatively homogenous genetic populations, compared to humans, allow for more refined genetic analyses, which can then inform human studies. Our study explored the genetic architecture of non-contact ACL rupture in Labrador Retrievers and humans through genome-wide association studies (GWAS), polygenic risk score (PRS) modeling, and cross-species pleiotropy analysis. By linking findings from these two species, we aimed to uncover shared genetic risk factors and mechanisms.
What Did We Discover?
-
Heritability and Genetic Risk
Non-contact ACL rupture is a highly heritable disease. In dogs, heritability estimates ranged from 0.52 to 0.63 depending on the model, indicating a strong genetic basis. In humans, heritability estimates ranged from 0.30-0.33 derived from GWAS of single nucleotide polymorphisms (SNPs), highlighting a significant but comparatively lower genetic contribution. These findings underscore that while environmental factors like activity level and joint conformation play a role, genetic predisposition is a major determinant of ACL rupture risk.
- Shared Genetic Risk Across Species
Through cross-species pleiotropy analysis, we identified 16 genes shared between humans and dogs that contribute to ACL rupture risk. Notable among these were PTPRT and PTPRM, both associated with ligament structure and repair pathways. These shared genes cluster in genomic hotspots, reinforcing the hypothesis that conserved biological pathways underlie ligament injury risk in both species.
The extracellular matrix organization pathway was particularly enriched in both dogs and humans, highlighting its central role in ligament integrity.
- Polygenic Architecture and the Ligament Fatigue Mechanism
Non-contact ACL rupture is not generally the result of a single catastrophic overload event (Figure 1). Instead, the condition develops due to progressive ligament fatigue from repetitive stress. This fatigue mechanism is heavily influenced by polygenic factors. Thousands of small-effect genetic variants contribute to disease risk. In both species, we found evidence of negative selection pressure on deleterious variants, suggesting an evolutionary cost to ligament injury risk. Such insights challenge traditional narratives that frame ACL rupture as an isolated mechanical injury, emphasizing instead the interplay of genetic, anatomical, and biomechanical factors.
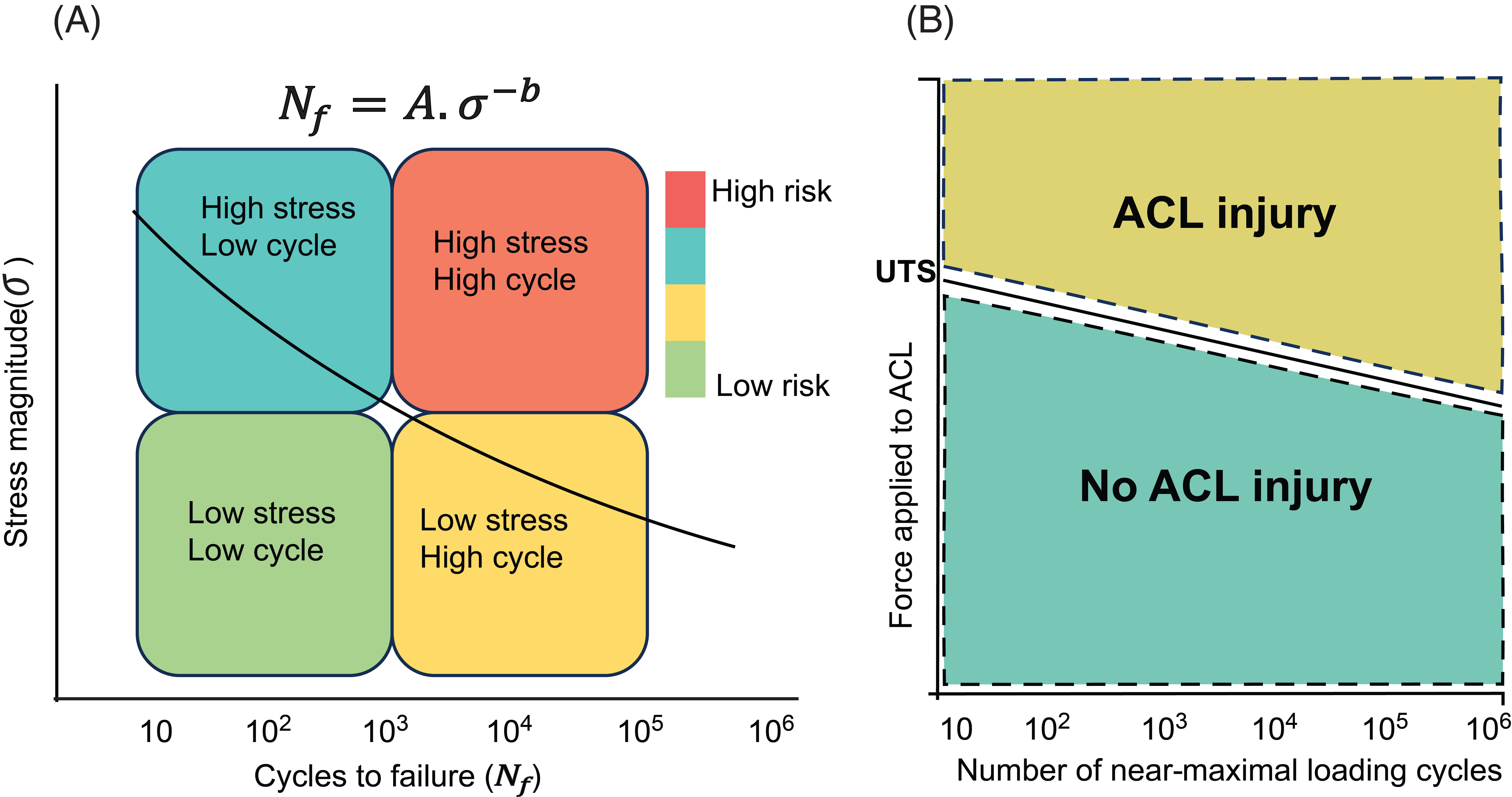
- Advancing Prediction With Polygenic Risk Scores
PRS values provide a promising tool for identifying individuals at high risk for ACL rupture. Using Bayesian regression models in dogs, we achieved considerable prediction accuracy. Bayesian approaches outperformed machine learning models like support vector machine and random forest, highlighting their robustness for prediction of polygenic disease risk. While existing GWAS data in humans provided moderate prediction accuracy, the integration of cross-species insights promises enhancement of human PRS models.
PRS prediction has the potential to revolutionize care by enabling early detection of high-risk individuals by screening. In humans, this could lead to tailored preventive care for athletes. In dogs, PRS values could guide breeding strategies and also inform veterinary care, especially for high-risk breeds like the Labrador Retriever, Rottweiler, and Newfoundland.
- Why Are Dogs the Perfect Model?
Dogs provide a powerful model for studying non-contact ACL rupture due to their unique genetic and clinical features:
Breed-Specific Risk. Certain breeds exhibit significantly higher ACL rupture risk due to genetic bottlenecks from selective breeding. For example, the Rottweiler and Labrador Retriever are predisposed breeds, whereas the Greyhound is highly protected.
High Linkage Disequilibrium (LD). Compared to humans, dogs have up to 100-fold larger LD blocks, making it easier to identify genomic regions associated with disease (5).
Translational Insights. Findings in dogs often translate well to humans, as seen with shared genetic pathways like extracellular matrix organization and skeletal muscle homeostasis.
- Implications for Precision Medicine
There is an urgent need to shift the paradigm for ACL rupture to provide impactful advances in the preventative care of affected patients who often develop disabling knee osteoarthritis. By integrating genetic insights with clinical data, our research shows the potential of precision medicine in managing ACL rupture patients. For humans, the PRS approach could guide interventions such as targeted physical therapy, knee strengthening programs, and early surgical consultations for high-risk individuals. For dogs, genetic screening could inform breeding programs and guide preventive care for at-risk breeds, reducing the societal burden of ACL rupture and associated conditions like osteoarthritis.
- A Paradigm Shift
Our findings redefine ACL rupture as a heritable, polygenic disease rather than a purely mechanical injury. By leveraging dogs as a translational model, we have taken significant steps toward unraveling the genetic basis of this complex condition. As with other polygenic conditions, integration of advanced computational tools, multi-omics data, and cross-species insights will be pivotal in advancing the field. Through collaboration across veterinary and human medicine, we can reduce the burden of ACL rupture and improve outcomes for patients of both species.
References
- E.E. Binversie et al., Canine ACL rupture: a spontaneous large animal model of human ACL rupture. BMC. Musculoskelet. Disord. 23, 116; 10.1186/s12891-021-04986-z (2022).
- M. Montalvo et al., What's my risk of sustaining an ACL injury while playing sports? A systematic review with meta-analysis. Br. J. Sports. Med. 53, 1003-1012; 10.1136/bjsports-2016-096274 (2019).
- M. Kaynak, F. Nijman, J. van Meurs, M. Reijman, D.E. Meuffels, Genetic variants, and anterior cruciate ligament rupture: a systematic review. Sports. Med. 47, 1637–1650; 10.1007/s40279-017-0678-2 (2017).
- T.H. Witsberger, J.A. Villamil, L.G. Schultz, A.W. Hahn, J.L. Cook, Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am Vet Med Assoc. 232, 1818-1824; 10.2460/javma.232.12.1818 (2008).
- B. Sutter et al., Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome. Res. 14, 2388-2396; 10.1101/gr.3147604 (2004).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in