In the early 1910s, the English bacteriologist Frederick Twort found that Mycobacterium paratuberculosis the causative agent of Johne’s disease, a chronic enteritis in cattle, was difficult to culture in vitro unless dried, inactivated human tubercle bacilli (Mycobacterium tuberculosis) were added to the growth medium. The “essential substance” associated with M. tuberculosis was later found to have a high affinity for iron and to specifically stimulate growth of mycobacteria at very low concentrations. These low-molecular-weight iron-chelating molecules were named ‘mycobactins’ (Fig. 1A, B) and belong to the so-called “siderophores” (iron carriers) produced by bacteria and fungi to acquire iron. For a long time, it was unknown how mycobactins are secreted by mycobacteria. In 2013, our laboratory showed that the small membrane proteins MmpS4 and MmpS5 are required for siderophore secretion by the human pathogen M. tuberculosis and that deletion of both genes drastically reduces its virulence in mice (Wells et al., 2013). MmpS4 and MmpS5 are associated with the efflux pumps MmpL4 and MmpL5 and constituted the first known components of the siderophore secretion system in M. tuberculosis. Surprisingly, the mmpS4 and mmpS5 double deletion mutant is hypersensitive to mycobactin even at nanomolar concentrations in contrast to the parent strain (Jones et al., 2014). We termed this toxicity of siderophores in the presence of heme as an alternative iron source ‘siderophore-poisoning’. Subsequently, deep sequencing experiments of M. tuberculosis transposon mutagenesis libraries (TnSeq) grown on different iron conditions showed a similar phenotype for a mycobacterial core gene rv0455c (Zhang et al., 2020). Rv0455c is a 14-kDa secreted antigen in many reports and its homologs share a conserved domain of unknown function in all mycobacterial species. In this study, we provided experimental evidence that Rv0455c is required for siderophore secretion by M. tuberculosis using a combination of genetic and biochemical approaches. In all experiments, the rv0455c deletion mutant exhibited similar phenotypes as the known siderophore secretion mutant lacking the mmpS4 and mmpS5 genes, including its extraordinary sensitivity to exogenous mycobactin (Fig. 1C). Although siderophore secretion by M. tuberculosis is not fully understood yet on a molecular level (Fig. 1D), the unusual structure of a Rv0455c homolog as shown in our study provides a solid basis for future mechanistic studies. Importantly, the rv0455c deletion mutant is strongly attenuated in mice, demonstrating that single proteins required for siderophore secretion exist in M. tuberculosis and validating siderophore secretion as a target for novel anti-tuberculosis drugs.
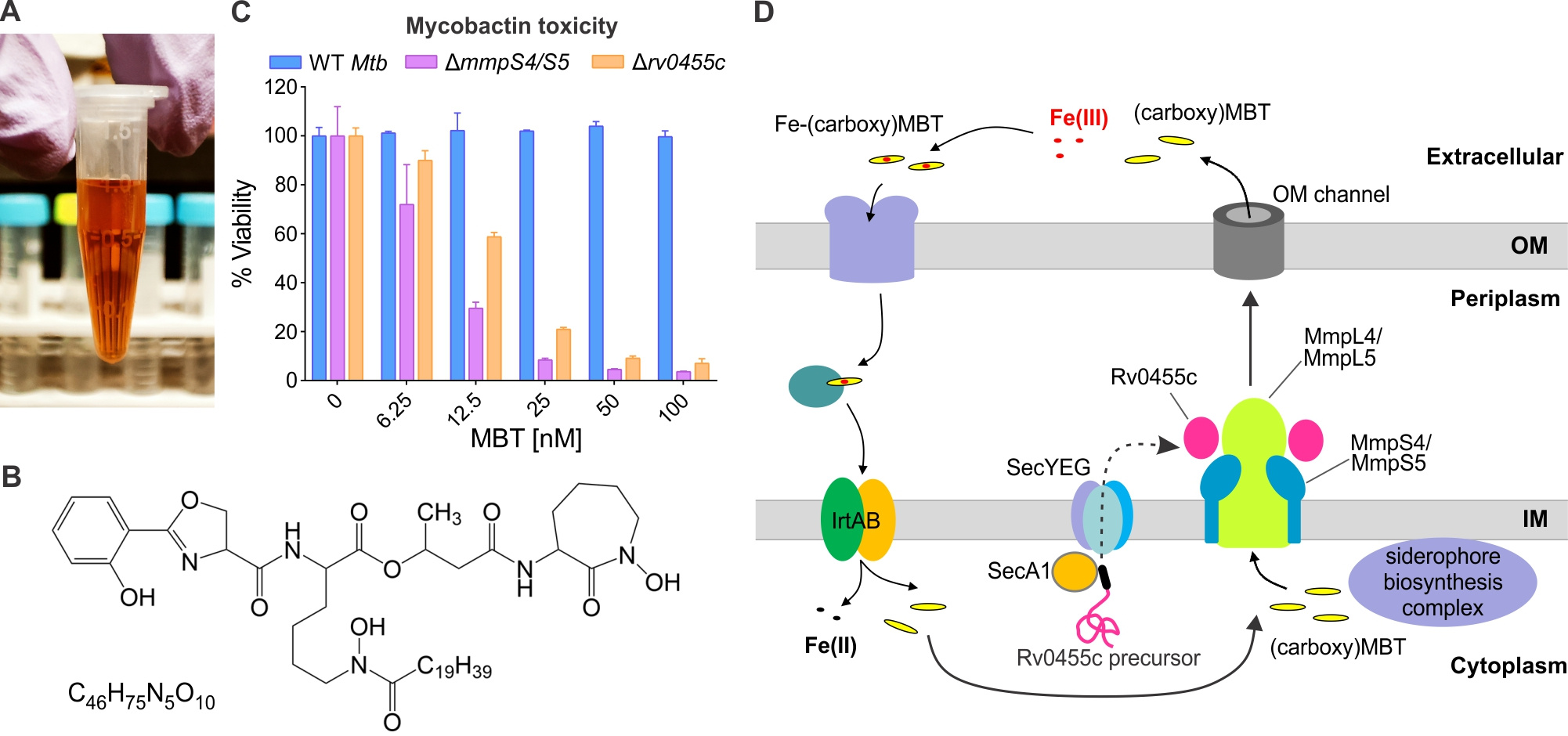
Figure 1. Siderophore secretion by Mycobacterium tuberculosis. (A) Isolated mycobactin (MBT) complexed with iron (1 mg/mL Fe-MBT ethanol solution). (B) Chemical structure of a mycobactin (C46H75N5O10). (C) Mycobactin (MBT) toxicity test for the wild-type M. tuberculosis (Mtb), the siderophore secretion mutant ΔmmpS4/S5 and the Δrv0455c mutant. (D) Model of siderophore secretion and recycling in M. tuberculosis. The periplasmic Rv0455c protein with the MmpS4/L4-MmpS5/L5 efflux pumps are required for (carboxy)mycobactin secretion in M. tuberculosis.
Full details can be found in our recent publication:
https://doi.org/10.1038/s41467-022-29873-6
References:
Jones CM, Wells RM, Madduri AV, Renfrow MB, Ratledge C, Moody DB, Niederweis M (2014) Self-poisoning of Mycobacterium tuberculosis by interrupting siderophore recycling. Proc Natl Acad Sci U S A 111:1945-50
Wells RM, Jones CM, Xi Z, Speer A, Danilchanka O, Doornbos KS, Sun P, Wu F, Tian C, Niederweis M (2013) Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis. PLoS Pathog 9:e1003120
Zhang L, Hendrickson RC, Meikle V, Lefkowitz EJ, Ioerger TR, Niederweis M (2020) Comprehensive analysis of iron utilization by Mycobacterium tuberculosis. PLoS Pathog 16:e1008337.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in