Solvent induced amyloid polymorphism and the uncovering of the elusive class 3 amyloid topology
Published in Materials, Protocols & Methods, and Cell & Molecular Biology
Amyloid forming peptides (APRs)
Amyloids are fibrillar aggregates of polypeptides with a high content of β-sheet secondary structure. Although amyloids formed by misfolded proteins have been implicated in more than fifty human diseases, including Alzheimer’s and Parkinson’s diseases, research is sheding light on an increasing number of of biological processes in which amyloid formation is utilized in vital biological functions. One such example of functional amyloids is the storage of some hormone peptides, which are stored in a compact amyloid form and immediately released upon a pH change due to the reversible nature of these amyloids. Aggregation prone regions (APRs) are short, highly amyloidogenic oligopeptides that can initiate the amyloid formation of longer sequences. Since these peptides are also capable of forming amyloids themselves, they can be used as simple, easier to handle model systems of amyloid formation. Our group is working on the structural aspects of amyloid formation, studying full-length polypeptides (e.g. glucagone) and proteins (e.g. TTR), as well as on some of their APRs to better understand the sequence- and condition dependence of amyloid formation.
Amyloid-like crystal structures
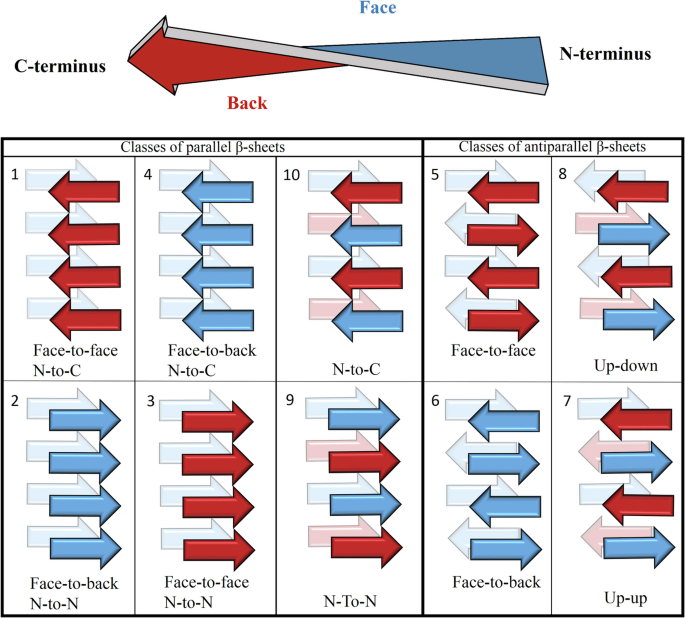
In addition to amyloid filaments and fibers, some APRs also form crystals too that allow structure determination by X-ray diffraction. In these crystals, the polypeptide chains assemble into tightly packed β-sheets with interacting side chains, resulting in steric zippers. These interfaces are similar to those observed in the amyloid cores of full-length proteins. APR crystal structures were originally classified into 8 topological classes, based on the relative orientation of the adjacent β-strands. Among the hundreds of determined crystal structures of APRs, composed of proteinogenic amino acids, there are multiple examples for most classes, except for class 3, for which the very first example is reported here. By studying the APR members of the glucagon polypeptide family, we have identified the LYIQWL hexapeptide, which surprisingly gave a large pool of polymorphic amyloids, among which both parallel and antiparallel β-sheet-containing amyloids were identified. On the road of the rational design of amyloids, we wanted to make amyloids composed of the parallel β-sheets more favorable, by completing the Trp5Asn (W5N) mutation.
Crystal structures

The remarkable diversity of LYIQWL amyloid crystals of has revealed a new polymorphic structure, when crystallized from 10 v/v% ethanol at 37°C after the removal of residual acid (TFA) contamination. Similar to the amyloidic form observed in 30 v/v% ethanol, this crystal also contains parallel β-sheets, but with different molecular architecture. Moreover, from the same solution, but at 4 °C, amyloids with antiparallel β-sheets grew, that were identical to those obtained from pure water. In contrast to the structural diversity of LYIQWL, the mutant LYIQNL gave only parallel β-sheet amyloids. From pure ethanol, we obtained the first pure class 3 amyloid nanocrystal, and thus have found the missing element! Although the mutation reduced the polymorphic diversity, LYIQNL from pure water yielded a class 4 amyloid. Although this is a different class of amyloid, the interface formed is similar to that observed for class 3.
Polymorphism in the solution
Since amyloid formation and crystallization are similar processes however with distinct differences, we wanted to determine whether the ethanol concentration-dependent amyloid polymorphism observed in the solid state is also present in the solution? We have acquired spectroscopic data (e.g. ECD, FT-IR) on the early phase and yet soluble amyloids and confirmed that the main structural features of the amyloid classes are present from the early phase of amyloid formation. In other words, the self-association differences induced by ethanol concentration and thus the amyloid formation pathways of class 3 and class 4 topologies are different from the earliest and form the smallest amyloid seeds. This supports that the characteristic differences observed in the mature crystals are present from the very beginning of the process. However, a higher degree of polymorphism of the solution phase cannot be excluded. The remarkable environment-dependent structural feature of APRs shows that they are ideal models to study the polymorphism of amyloids and to fine-tune their design.
Outlook
Methods based on artificial intelligence (AI) revolutionized the approach to structure prediction of globular proteins. This achievement would not have been possible without the large amount (>220 000) of structural information available on globular proteins and their complexes. However, structure prediction of amyloids remains challenging, due to the small number of 3D amyloid structures (<1000) determined to date and their high propensity for polymorphism. In contrast to evolutionarily selected globular proteins, an amyloidogenic sequence (e.g. APRs) can give rise to several different amyloid forms depending on the molecular conditions. We believe that by studying the structural and the polymorphic nature of APRs, we can elucidate how and what determines the final 3D amyloid structures, which will help to better design of these biocompatible, responsive nanomaterials of versatile applicability.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in