Synchrotron radiation X-ray microscopy techniques: eyes to see the nanomaterials and subcellular architectures in intact single cells
Published in Protocols & Methods

A cell, as the basic structural and functional unit of the life, is the main site where nanomedicines exert their functions and bioactivities. The interactions of nanomedicines/nanoparticles (NPs) with cells, including the cellular behaviors of NPs and morphological alterations of subcellular structures, determine the medical efficacy and biosafety of NPs. For example, the endocytosis and lysosomal escape are decisive for the efficient targeting and therapy. Enhanced cellular uptake of nanovaccines by antigen-presenting cells is one of the key steps in immune potentiation. Instability and rupture of lysosomal membrane could be related to the cytotoxicity response (e.g., inflammation). The underlying principles and mechanisms of nano-cell interactions is needed to be elucidated, which will guide the safe-by-design of nanomedicines and eventually support the clinical translation.
In situ analysis of subcellular structures in intact single cells and intracellular behaviors of NPs without labeling can provide detailed and accurate information to understand nano-cell interactions. However, there are great challenges for in situ label-free analysis of single cells at nanometric resolution due to the complexity of cellular structures and dynamic changes in chemical forms of NPs. With the development of light sources, optics and detectors, X-rays, especially the synchrotron radiation X-rays, provide great opportunities to analyse intact single cells at high resolution without labeling. Synchrotron radiation X-rays can penetrate deeply inside specimens and interact with matters to produce absorption, phase, and fluorescence signals without labeling, providing information on the spatial distribution, volume, number, density, and even the valance state of NPs, as well as the morphological changes of cells or tissues. Synchrotron radiation-based techniques have multiple advantages of label-free, in-situ, high resolution, element-specific, quantitative analysis, and simple sample preparation, which enable the study of the biological behavior and fate of NPs in cells or tissues with native or near-native states.
For more than two decades, Prof. Chunying Chen’s group at National Center for Nanoscience and Technology of China has been devoting to developing methods for the analysis of the nano-bio interactions and at the forefront of analytical methods innovation using synchrotron radiation light source facilities. With the synchrotron radiation-based techniques, our group quantitatively analyzed the interaction of nanoparticles with macromolecules (proteins, phospholipids, etc.) at the nanoparticle-biological interface and qualitatively characterized the chemical behavior of NPs in organisms1-10. Using synchrotron-radiation based techniques, we have successfully broken through the technical barriers of in situ, label-free analysis of NPs in complex biological systems, paving the way for a new research paradigm in the cross-scale analysis of NPs at molecular, interface, cell and tissue levels.
In our recent article published in Nature Protocols, we summarized the technical details of synchrotron radiation soft and hard X-ray microscopy including full-field transmission X-ray microscopy (TXM) and scanning transmission X-ray microscopy (STXM). Our protocol presented a comprehensive method of 3D soft X-ray TXM, 3D hard X-ray TXM and 2D soft X-ray STXM, realizing the in situ, label-free and 2D/3D imaging of cellular structures and NPs, as well as the chemical transformations of NPs (Fig. 1a). The protocol has been successfully implemented at beamline 4W1A of Beijing Synchrotron Radiation Facility (BSRF), BL08U1A of Shanghai Synchrotron Radiation Facility (SSRF) and BL07W of the National Synchrotron Radiation Laboratory (NSRL).
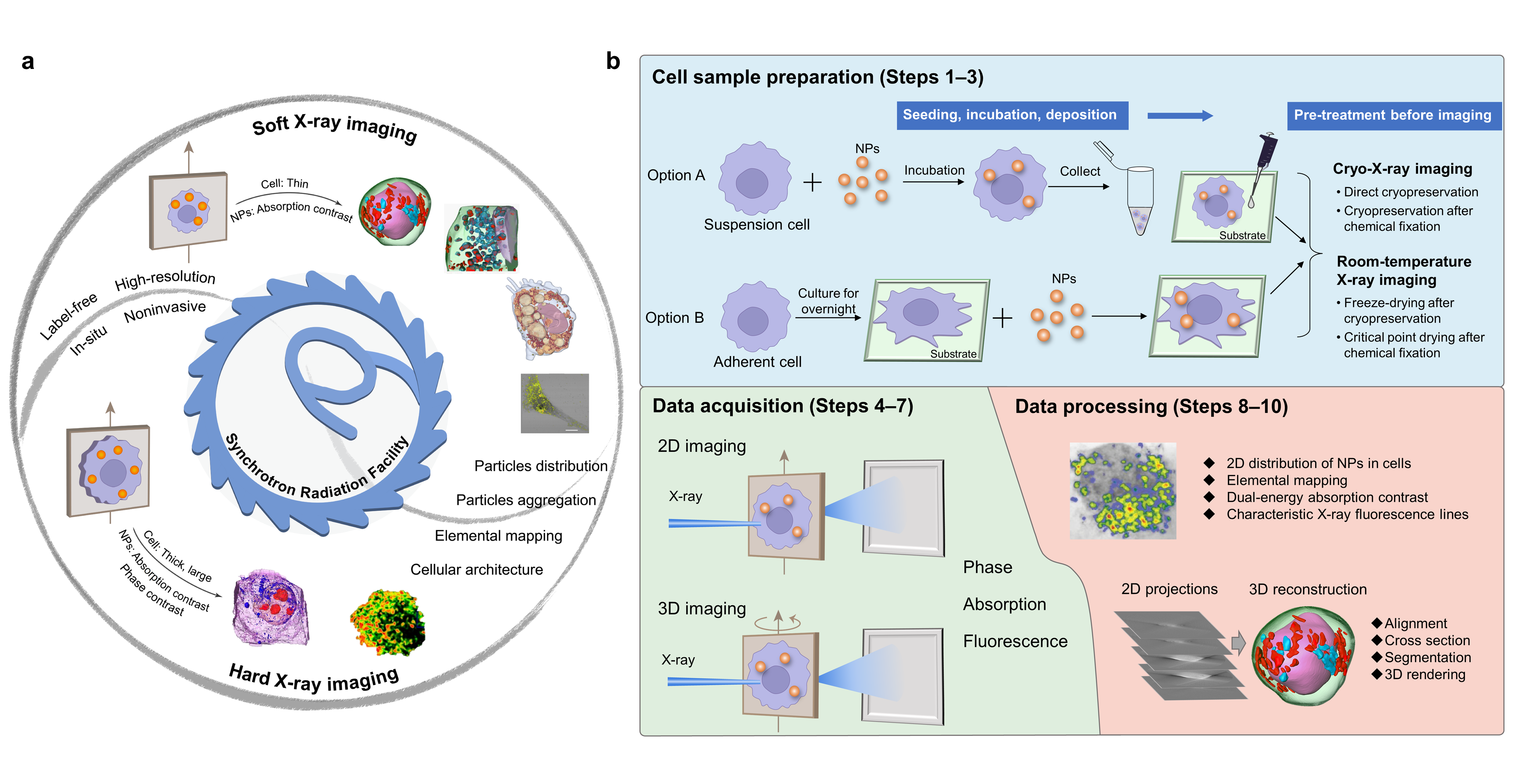
Fig. 1. Soft and hard X-ray imaging of NPs and subcellular architecture in intact cells based on synchrotron radiation facilities and the overall procedure
We proposed an optimized workflow including beamline selection, cell sample preparation, data acquisition and analysis (Fig. 1b). Among them, beamline selection is a key priority, which is decisive for the following procedures. By taking several model NPs as examples, we provided a practical guideline for users to select proper X-ray beamlines based on the types of cells and NPs, as well as the research purpose. Besides, we proposed four sample preparation procedures based on the imaging conditions of the beamline and different users’ demands. Moreover, data acquisition and processing are described in detail, which was sketchily mentioned or ignored in the related protocols previously published.
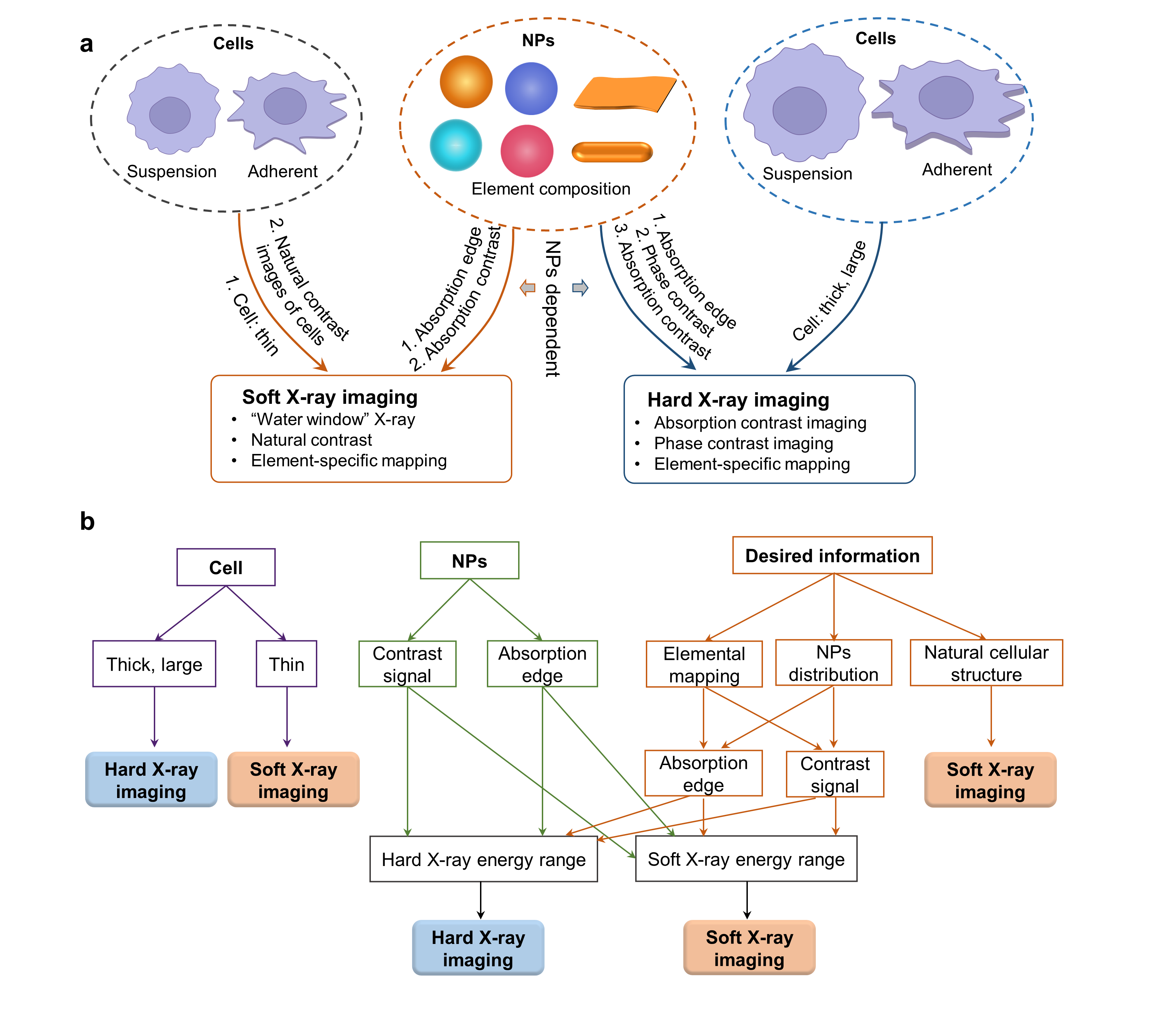
Fig. 2. Guideline for beamline selection from soft and hard X-ray imaging
Beamline selection: Cell types and NPs’ compositions are key factors determining the options from soft and hard X-ray imaging beamlines (Fig. 2). If the cell is thin (< 10 mm), or 2) element-specific information of intracellular NPs is preferred and the absorption edge of the element of NPs is in the soft X-ray energy range, or 3) NPs have high absorption contrast at soft X-ray energy which can be distinguished from cellular compositions, or 4) the natural cellular structure is the desired information, soft X-ray imaging is suitable. Hard X-ray imaging is the preferable choice if 1) the cell is large and thick, or 2) the absorption edge of the element is in the hard X-ray energy region, or 3) NPs have distinct absorption/phase contrast at hard X-ray range.
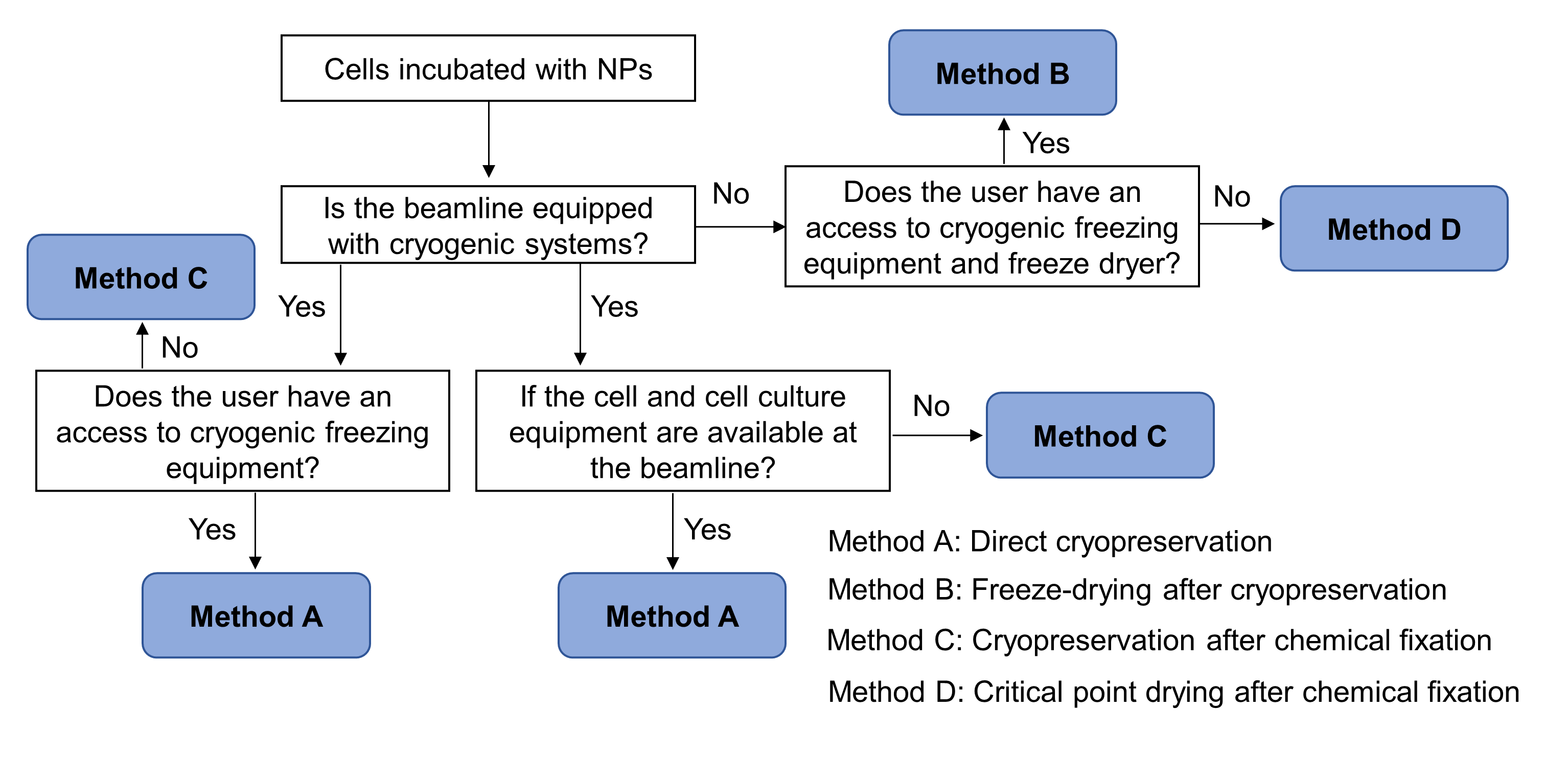
Fig. 3. A decision-making flow chart for preservation of cell-NPs samples, based on the imaging conditions of the beamlines and different users’ demands
Sample preparation: Incubation of cells with NPs and sample preservation before imaging are the two steps for sample preparation. The incubation method varies with cell types (adherent or suspension or primary): the differences are the use order of the substrate, types of the substrate and the time required. After the incubation, cell samples should be preserved to meet the sample requirement for the selected X-ray imaging beamline. Based on the imaging conditions of the beamlines and different users’ demands, we proposed four preservation methods: direct cryopreservation, freeze-drying after cryopreservation, cryopreservation after chemical fixation and critical point drying after chemical fixation. The four methods have their own advantages and limitations, satisfying different beamline users’ requirements. Fig. 3 summaries a decision-making flowchart for preservation of cell-NPs samples.
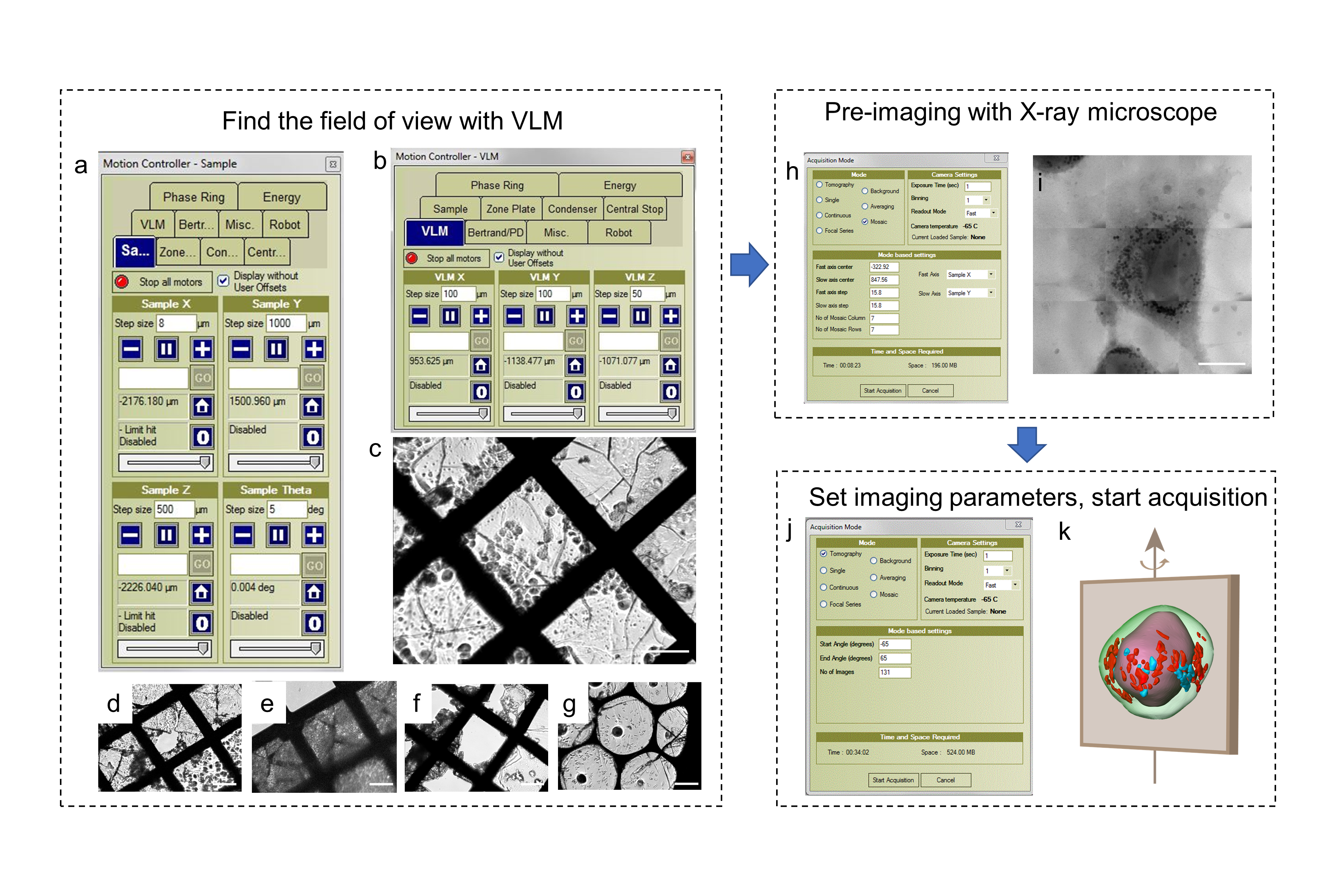
Fig. 4. Data acquisition procedure of cryo-soft X-ray nano-CT imaging performed at beamline 07W of NSRL
Data acquisition and analysis: Detailed standard operating procedures of the image acquisition vary with the beamline selected (Fig. 4). Training and expertise are required to accomplish the data acquiring independently. Data processing methods are dependent on the raw data type and research purpose (2D or 3D, elemental or structure information, absorption or fluorescence or phase signal). 2D data can be processed by reducing the background noise and graphing images with the corresponding software. 3D images can be obtained via background reduction, alignment of the tilt series, 3D tomography reconstruction, segmentation, and rendering. The protocol describes the overall steps by taking beamline BL08U1A of SSRF, beamline BL07W of NSRL and 4W1A of BSRF as examples for 2D STXM, 3D cryo-soft X-ray nano-CT and hard X-ray nano-CT, respectively.
The protocol offers a systematic research strategy for investigating the subcellular structures and intracellular behaviors of NPs in situ and label-free, based on synchrotron radiation X-ray microscopy. The research strategy not only aims to elucidate the fundamental principles and mechanisms underlying nano-cell interactions, but also serves as a powerful tool for exploring the alterations in cellular components, the homeostasis of bioelements, the pharmacology and toxicology evaluation of metal-based materials. This protocol holds immense potential in multidisciplinary fields, including chemical metrology, cell biology, nanomedicine and materials science, etc.
Link to the full article: https://doi.org/10.1038/s41596-023-00902-y
References:
- Mingjing Cao, Chunying Chen*, et al. Molybdenum derived from nanomaterials incorporates into molybdenum enzymes and affects their activities in vivo. Nature Nanotechnology, 2021, 16: 708−716.
- Guofang Zhang, Yong-Tang Zheng*, Hongchang Li*, Chunying Chen*, Liming Wang*, Yang Li*, et al. A nanomaterial targeting the spike protein captures SARS-CoV-2 variants and promotes viral elimination. Nature Nanotechnology, 2022, 17: 993-1003.
- Xiaoyu Wang, Xuejing Cui*, Chunying Chen*, et al. Peripheral nerves directly mediate the transneuronal translocation of silver nanomaterials from the gut to central nervous system. Science Advances, 2023, 9(27): eadg2252.
- Mingjing Cao, Yaling Wang*, Chunying Chen*, et al. Advanced light source analytical techniques for exploring the biological behavior and fate of nanomedicines. ACS Central Science, 2022, 8(8): 1063-1080.
- Chunying Chen, Yuliang Zhao*, et al. Advanced nuclear analytical and related techniques for the growing challenges in nanotoxicology. Chemical Society Reviews, 2013, 42(21): 8266-303.
- Chunyu Zhang, Chunying Chen*, Yaling Wang*, et al. Multifunctional nanoprobe for 3D nanoresolution imaging of intact cell HER2 protein with hard X-ray tomography. Analytical Chemistry, 2023, 95(4): 2129-2133.
- Liming Wang, Xiaochun Wu*, Chunying Chen*, et al. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: Understanding the reduced damage in cell membranes. Journal of the American Chemical Society, 2013, 135: 17359-17368.
- Yaling Wang, Ye Liu*, Chunying Chen*, et al. Engineering a self-navigated MnARK nanovaccine for inducing potent protective immunity against novel coronavirus. Nano Today, 2021, 38: 101139.
- Liming Wang, Binhua Lin*, Ruhong Zhou*, Chunying Chen*, et al. Stability of ligands on nanoparticles regulating the integrity of biological membranes at the nano–lipid interface. ACS Nano, 2019, 13: 8680-8693.
- Liming Wang, Chunying Chen*, et al. Use of synchrotron radiation-analytical techniques to reveal chemical origin of silver-nanoparticle cytotoxicity. ACS Nano, 2015, 9: 6532-6547.
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in