Synergistic induction of mitotic pyroptosis and tumor remission by inhibiting proteasome and WEE family kinases
Published in Cancer, Cell & Molecular Biology, and Pharmacy & Pharmacology

Background
Induction of mitotic catastrophe (MC) is considered as an attractive strategy to selectively kill cancer cells1,2. The reported modes of cell death under MC are often attributed to intrinsic apoptosis3,4. Whether pyroptosis, a newly defined mode of cell death5, is involved in MC remains unreported. During mitosis phase (M-phase), both transcription6 and translation7 of mRNAs are globally repressed, the proteasome-mediated protein degradation thus plays important roles in regulating M-phase8. Bortezomib (BTZ) is the first proteasome inhibitor approved to treat human cancers, like multiple myeloma and mantle cell lymphoma, but primary resistance to BTZ in solid tumors limits its clinic use9. To date, whether and how proteasome inhibitor affected the fates of cells in M-phase remains unexplored. The inhibitors of G2 checkpoint activators, like WEE family kinases and CHK1/2 display high toxicity and low efficacy10, resulting in termination of further clinical development. Whether these inhibitors can synergize with proteasome inhibitor in killing cancer cells is still unknown.
Our findings
In this study, we find that inhibition of proteasome by BTZ treatment or silencing of PSMC5, a subunit of 19S regulatory particle of proteasome, causes multi-polar spindle formation, G2- and M-phase arrest, and consequent pyroptosis in M-phase (designated as mitotic pyroptosis). Mechanism studies show that proteasome inhibitor induces mitotic catastrophe in a form of mitotic pyroptosis via cGAS-caspase-3-GSDME cascade. Further investigations reveal that inhibitor of WEE1/PKMYT1 (PD0166285), but not inhibitor of CHK1 or CHK2, abrogates the BTZ-induced G2-phase arrest, thus exacerbates the role of BTZ in inducing mitotic arrest and pyroptosis. Compared to BTZ or PD0166285 monotreatment, combined BTZ and PD0166285 treatment (named BP-Combo) selectively kills cancer cells and significantly lessens the IC50 of both BTZ and PD0166285. Various mouse models reveal that BP-Combo shows much stronger inhibition on tumor growth than BTZ or PD0166285 monotreatment, and BP-Combo also represses tumor metastasis and prolongs survival of tumor-bearing mice without obvious toxicity on normal tissues.
Conclusions
These findings disclose the role of proteasome inhibitor in inducing pyroptosis, characterize intrinsic pyroptosis as a new death form of MC, and identify the combined inhibition of proteasome and WEE family kinases as a promising strategy to specifically eliminate cancer cells, which is of great clinic significance and merits further clinical trials in cancer therapy (Figure 1).
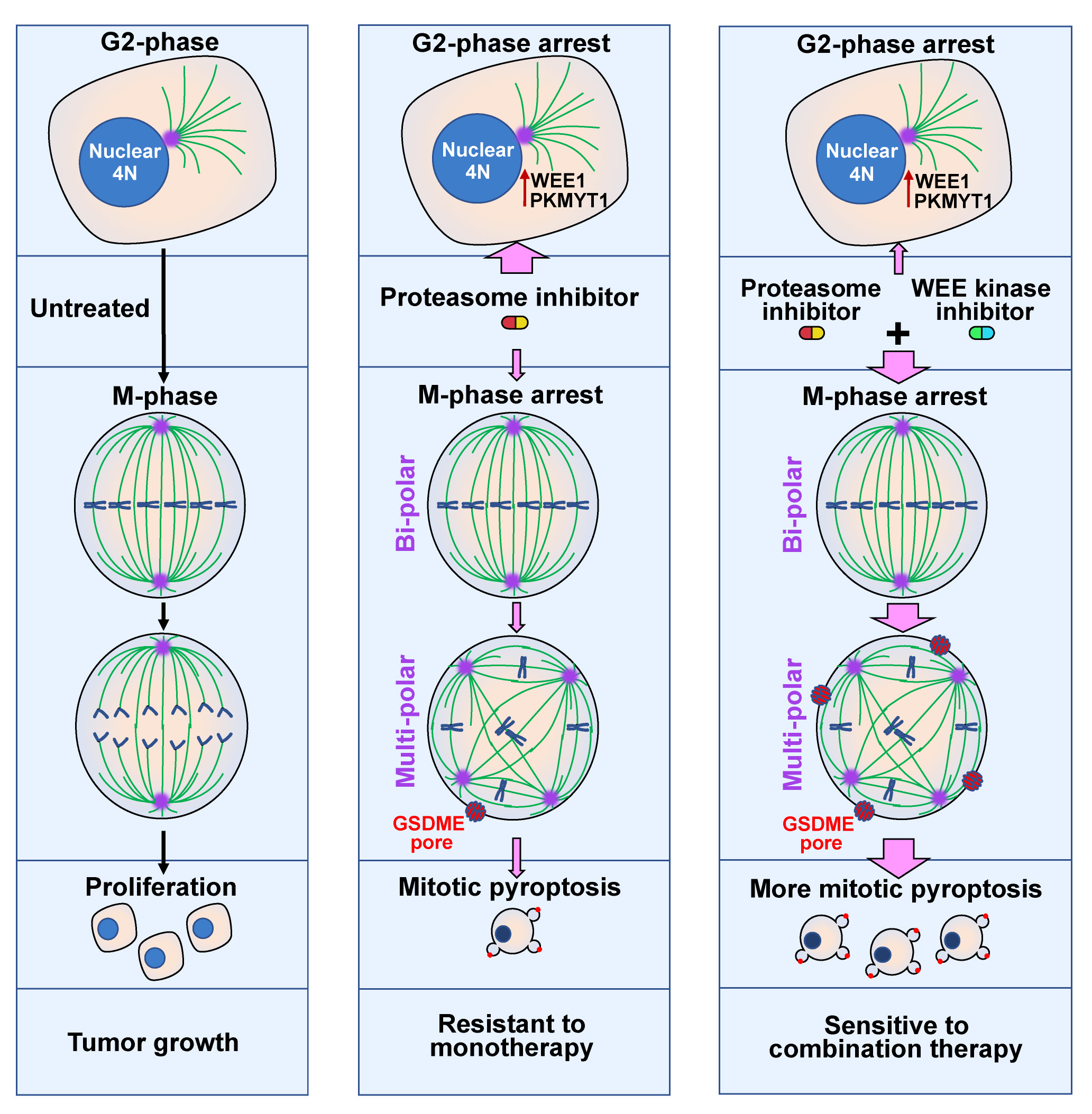
Figure 1. The working model of synergistic interaction between proteasome and WEE kinase inhibitors.
For more details of our experimental methodology and results, please read our newly published paper in Signal Transduction and Targeted Therapy (DOI: 10.1038/s41392-024-01896-z, https://rdcu.be/dNvJY).
References
1 Vitale, I., Galluzzi, L., Castedo, M. & Kroemer, G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 12, 385-392 (2011).
2 Dominguez-Brauer, C. et al. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 60, 524-536 (2015).
3 Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486-541 (2018).
4 Hellmuth, S. & Stemmann, O. Separase-triggered apoptosis enforces minimal length of mitosis. Nature 580, 542-547 (2020).
5 Shi, J., Gao, W. & Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 42, 245-254 (2017).
6 Palozola, K. C., Lerner, J. & Zaret, K. S. A changing paradigm of transcriptional memory propagation through mitosis. Nat. Rev. Mol. Cell Biol. 20, 55-64 (2019).
7 Tanenbaum, M. E., Stern-Ginossar, N., Weissman, J. S. & Vale, R. D. Regulation of mRNA translation during mitosis. Elife 4 (2015).
8 Min, M. & Lindon, C. Substrate targeting by the ubiquitin-proteasome system in mitosis. Semin. Cell Dev. Biol. 23, 482-491 (2012).
9 Manasanch, E. E. & Orlowski, R. Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 14, 417-433 (2017).
10 da Costa, A., Chowdhury, D., Shapiro, G. I., D'Andrea, A. D. & Konstantinopoulos, P. A. Targeting replication stress in cancer therapy. Nat. Rev. Drug Discov. 22, 38-58 (2023).
Follow the Topic
-
Signal Transduction and Targeted Therapy

This is an international, peer-reviewed, open-access journal publishing articles related to signal transduction in physiological and pathological processes, alongside signal transduction-targeted therapeutics in the form of biological agents and small molecular drugs used to treat human diseases.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in