Targeting cell plasticity in cancer and beyond
Published in Cancer

This cancer community platform will probably acknowledge how important cancer cell dissemination is in the body and how it leads to metastasises. Being confronted with a personal case in the family of someone with a tumour and resulting cancer metastasis, a situation which I daresay many readers may identify themselves with, this also became a personal matter, as with many topics in research. In this writer's opinion, many choices of pursuit in science have a rather personal motivation. It is almost disheartening to see that oncologists do not have many choices when it comes to metastasis treatment. In some cases invasive operative measures or radiation therapy can be applied, and in some cases chemotherapy can make a difference. However, ultimately around 90% of cancer deaths are due to cancer cell dissemination in the body.
Cancer cells can be highly proliferative or belong to the 'persister cancer cell niche'
Solid tumours are not just a mass of cancer cells, but have a complex microenvironment, are often infiltrated with immune cells, cancer-associated fibroblasts and others. Importantly, cancer cells themselves can (in a simplistic fashion) be divided into fast growing proliferating and slow growing more plastic cells. it is the latter where the persister cancer cell niche can be found, which are refractory to treatments that target cell proliferation. Unfortunately there the oncologists have little choice, as the weapons they have at their disposal target the proliferating cells. But importantly, in the minds of many people, this seems to be still the paradigm for new therapy: target cancer growth. But what about cancer dissemination?
Salinomycin sequesters iron in lysosomes and specifically kills cancer stem cells
Our story in the field began more than a decade ago with our first publication in the field describing how persister cancer cells (also often referred to as cancer stem cells) are addicted to iron1, making them more vulnerable to a process called ferroptosis2-3. This was interesting from a fundamental scientific point of view and also gave a new therapeutic premise as we found that iron is druggable in persister cancer cells with salinomycin and its potent derivative ironomycin. With this work we had a hard time with the reviewers in one point: salinomycin does not target the bulk of the tumour and has no to very moderate effects on overall tumour size, but it does selectively kill the cells leading to cancer cell dissemination. But this is precisely what makes this work special and we had to fight hard to make a point here. We later went on to develop more potent derivatives than ironomycin, including prodrugs that show efficacy on organoid models.
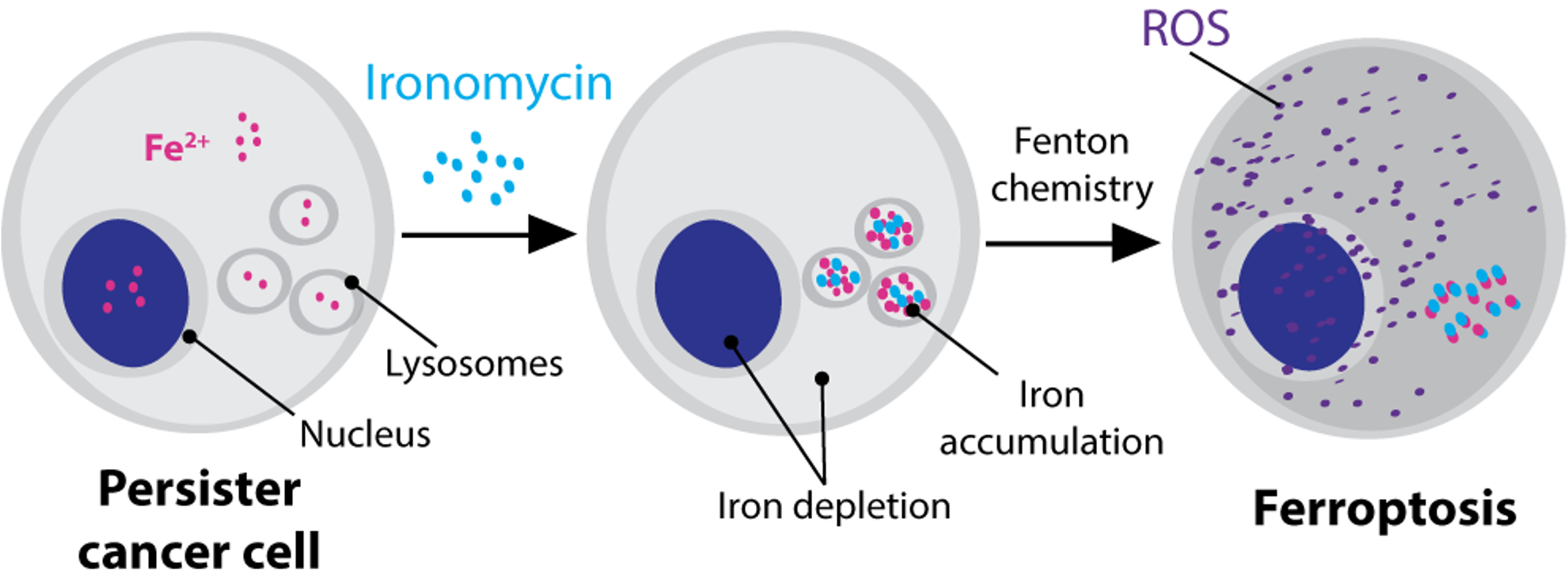
Taking this research further, we now developed a new molecule that has a lysosome-targeting domain fused with an iron-activating domain. This class of molecules can specifically activate lysosomal iron and preferentially kills persister cancer cells/ cancer stem cells in cell culture systems and in biopsies of fresh human tumours9.
CD44 is a cell plasticity regulator by taking up metals
This work led to our discovery that CD44 is a crucial protein in cancer cell plasticity by mediating iron endocytosis4. CD44 is a cell surface protein found in many cell types besides persister cancer cells. Importantly, it is used in many settings as a "marker" for activated cells, including immune cells such as dendritic cells, macrophages and T cells. Indeed, it has been known since 1990 that CD44 is the principle receptor for hyaluronic acid in cells5 and takes up the hyaluronates via endocytosis6. Our previous work on persister cancer cells made us wonder if CD44 could play a role in metal import in other cell types and regulate cell plasticity in a wide spectrum of cells. Thus, we studied this process in macrophages derived from primary monocytes isolated from blood. Using this system, we found that during inflammatory macrophage activation, cells predominantly take up metals via CD44, including copper, iron, calcium and magnesium8. Copper taken up by CD44 accumulates in mitochondria of cells, where it catalyses the interconversion of NAD(H) in a reaction using hydrogen peroxide. We investigated these processes further and developed a small molecule that can target mitochondrial copper to target cell plasticity. Indeed, this new molecule, called supformin, can interfere wit immune cell and cancer cell plasticity.
Supformin targets cell plasticity in inflammation and beyond
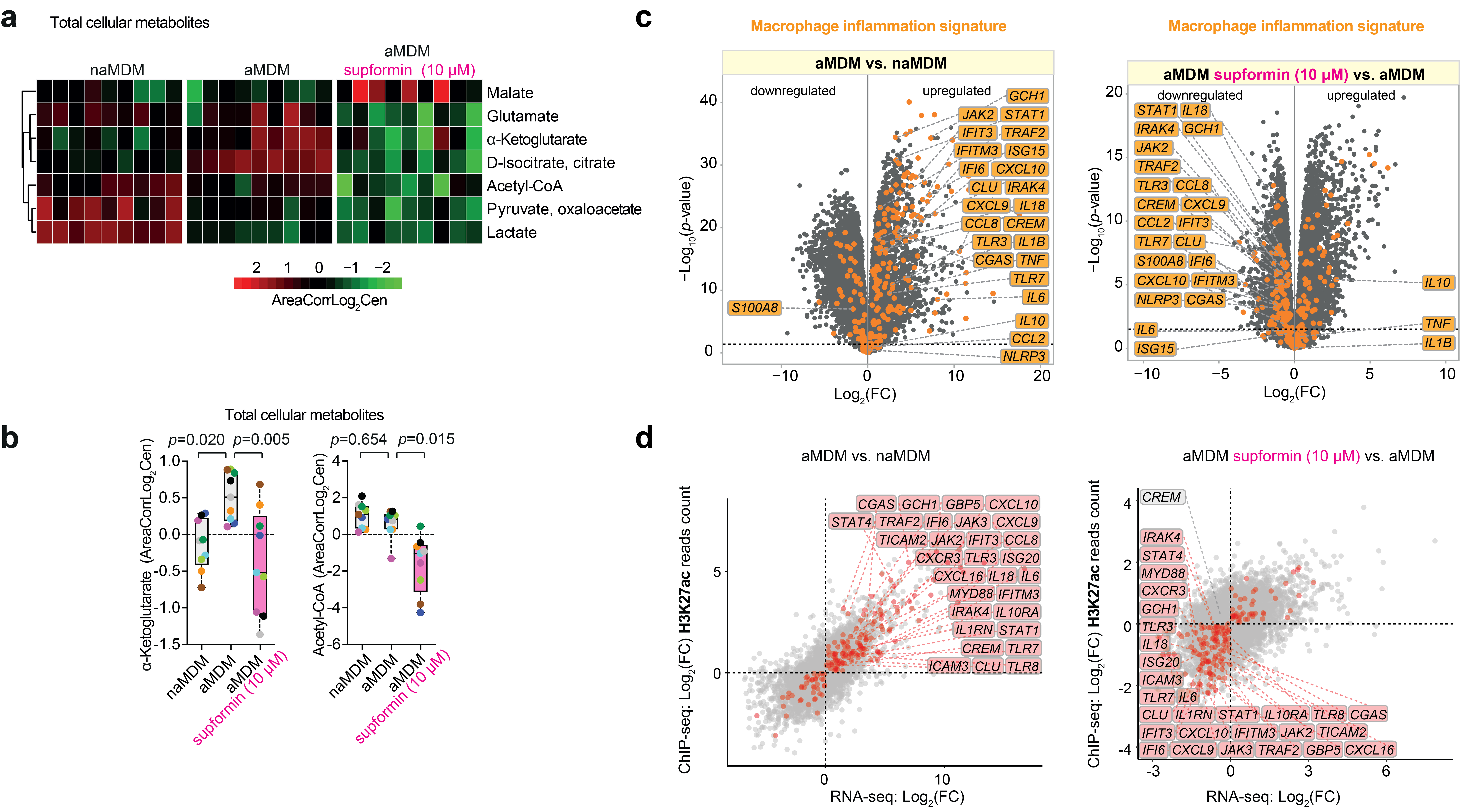
We found that supformin can reprogram cell metabolism and reduce total NAD(H) levels. Since these metabolites are crucial for many metabolic processes, including the Krebs cycle, levels of several metabolites were altered, of which acetyl-CoA and alpha-ketoglutarate stood out. These two metabolites are crucial for histone demethylation and acetylation in the cell nucleus, thus, changing their levels will ultimately lead to changes in the epigenetic landscape of the cell. Changes of the epigenetic landscape are inherent with changes in cell phenotype. Indeed, supformin treatment lead to epigenetic reprogramming into a less inflammatory state in macrophages.
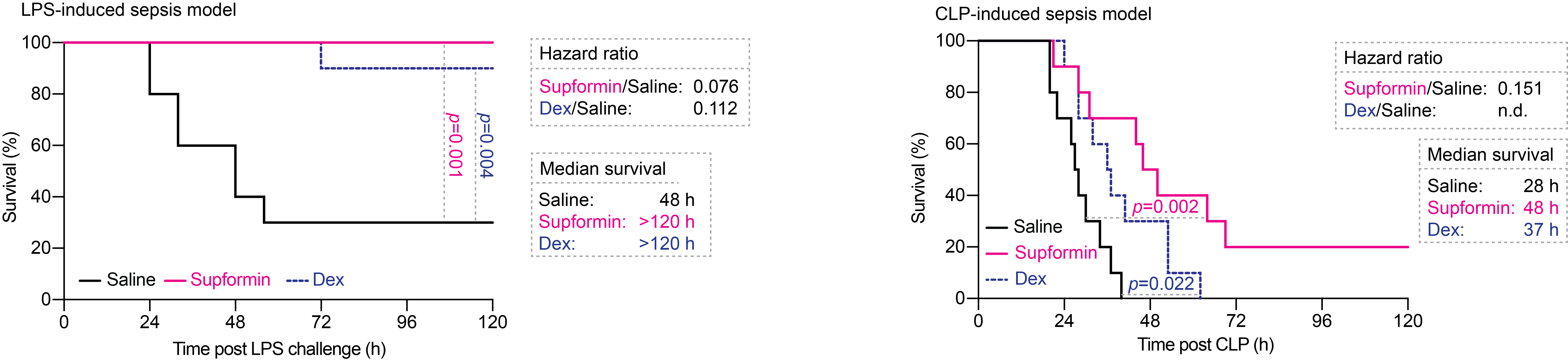
We also showed that supformin has an effect on cell plasticity in cancer cells undergoing epithelial-to-mesenchymal transition, an area of research we are focussing on now. Finally, supformin could rescue survival of mice in several models of sepsis in mice.
Collectively, our body of work now opens a new avenue and field: metal homeostasis in cell plasticity. This has profound implications in cancer research and beyond, as it can be applied to settings of inflammation and, as we think, way beyond that.
References
1 Nat Chem. 2017 Oct;9(10):1025-1033. doi: 10.1038/nchem.2778
2 Cell Metab. 2008 Sep;8(3):237-48. doi: 10.1016/j.cmet.2008.07.005
3 Cell. 2012 May 25;149(5):1060-72. doi: 10.1016/j.cell.2012.03.042
4 J Am Chem Soc. 2022 Jul 6;144(26):11536-11545. doi: 10.1021/jacs.2c03973
5 Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5
6 Cell. 1990 Jun 29;61(7):1303-13. doi: 10.1016/0092-8674(90)90694-a
7 J Cell Sci. 1993 Sep;106 ( Pt 1):365-75. doi: 10.1242/jcs.106.1.365
8 Nature. 2023 May;617(7960):386-394. doi: 10.1038/s41586-023-06017-4
9 Nature, 2025, May, doi: 10.1038/s41586-025-08974-4
Reference: Solier et al., A druggable copper-signalling pathway that drives inflammation, Nature, 2023, 617, 386–394, doi:10.1038/s41586-023-06017-4
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in