The AI-Driven CAR-T Design Arrives
Published in Cancer, Protocols & Methods, and Immunology

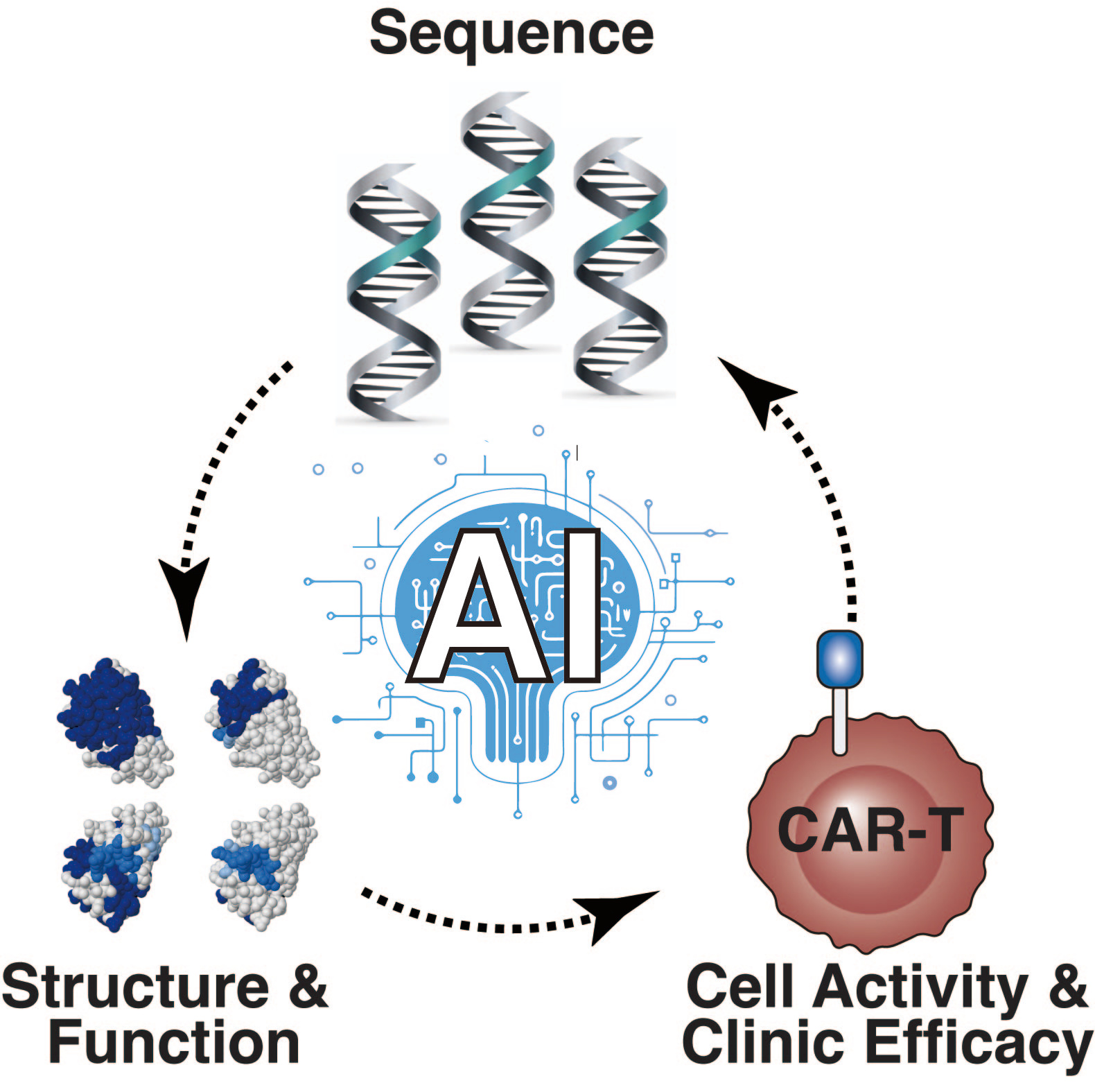
As we step into an era where artificial intelligence (AI) intersects with medical research, our team at ShanghaiTech University has taken a significant leap. Our recent publication in Cell Research on February 14, 2024, is more than just a paper; it's a testament to the potential of AI in revolutionizing cancer treatment through CAR-T therapy (1). Here’s the story behind our innovation, CAR-Toner, and its role in predicting and optimizing CAR tonic signaling.
The Birth of CAR-Toner
Our journey began with the recognition of two major bottlenecks in CAR-T cell therapy: the insufficient persistence of CAR-T (the lifespan of CAR-T cells inside of the patient's body) and CAR-T exhaustion after continuous CAR stimulation (2, 3, 4). The concept of tonic signaling – a weak but constitutive activation signal in CAR-T cells even in the absence of tumor antigens – emerged as a critical player in CAR-T cell activity and longevity.
CAR Tonic Signals, Understanding the Signal in the Absence of Antigen Stimulation
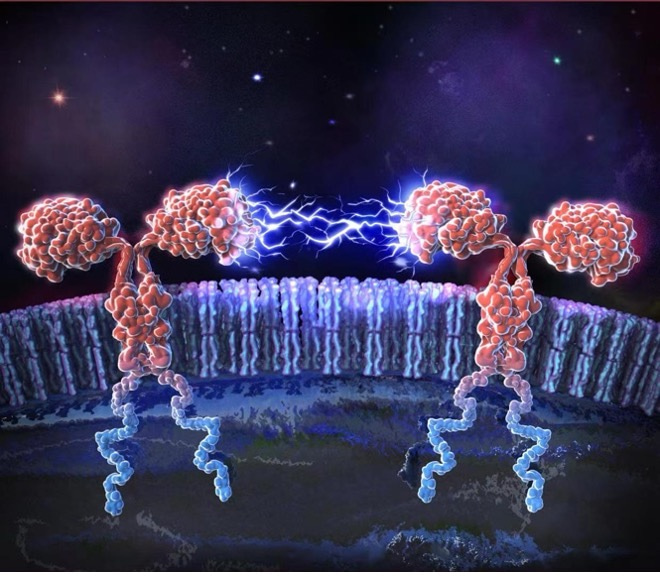
What initiates CAR tonic signals when there is no antigen stimulation? We discovered that the uneven distribution of positive charges on the CAR's surface prompts the clustering of CAR through electrostatic interactions between the receptors. This clustering is what sets off the tonic signaling in CAR-T cells. To quantify this phenomenon, we introduced a scoring system for Positively Charged Patches (PCP). This scoring system shows a near-linear positive correlation with the intensity of the CAR tonic signals, providing a measurable and predictable approach to understanding and potentially controlling the CAR tonic signaling strength (5).
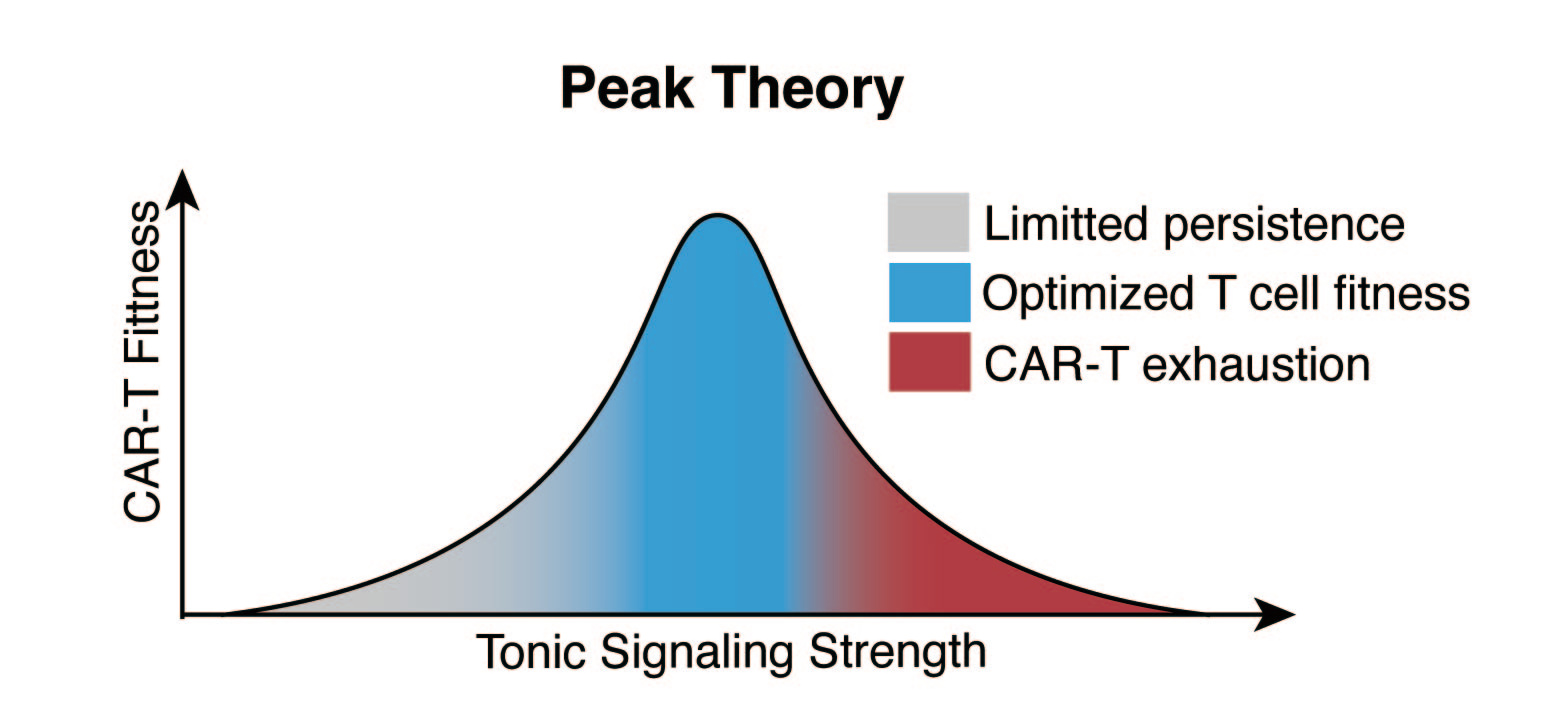
Peak Theory: Finding the Optimal Range
We further discovered a bell-shaped relationship between the intensity of tonic signals and the in-vivo functionality of CAR-T cells, which we introduced the "Peak Theory," suggesting that the tonic signal of CAR should be neither too strong nor too weak, as an insufficient tonic signal cannot maintain the steady-state proliferation of CAR-T cells, while an overly strong tonic signal leads to T cell functional exhaustion (4, 5, 6). Our in vivo studies indicate an optimal PCP range of 46-56, within which CAR-T cells exhibit the PEAK functional fitness in vivo, balancing robust anti-tumor activity with longevity and resistance to cell exhaustion(5).
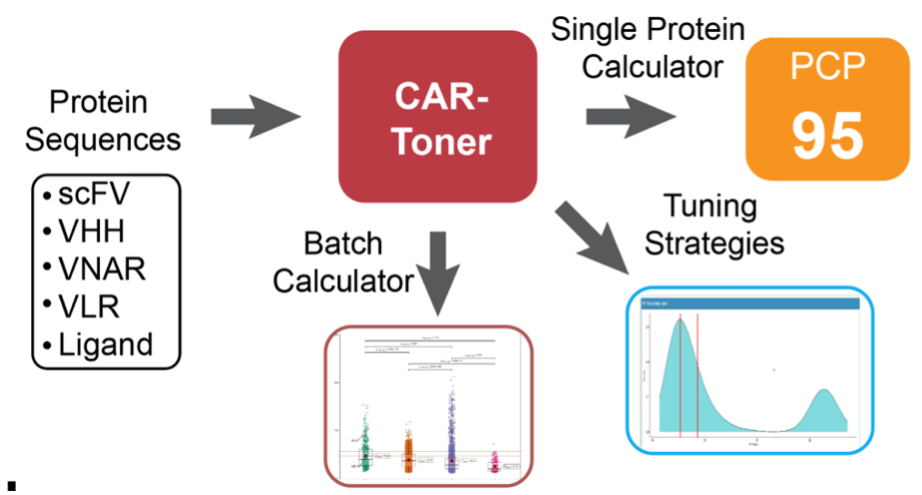
AI-Driven Design: Making the Leap
Computing PCP scores used to be complex, demanding a week to learn the process and relying on external server capacities that often crashed due to high demand. That's where AI stepped in, empowering us to create the CAR-Toner platform (http://cart-fitness.slst.shanghaitech.edu.cn/CAR-fitness/). It's a trifecta of capabilities: calculating PCP scores from CAR protein sequences, guiding CAR design optimizations, and evaluating a vast array of CAR constructs to identify the most promising design (1).
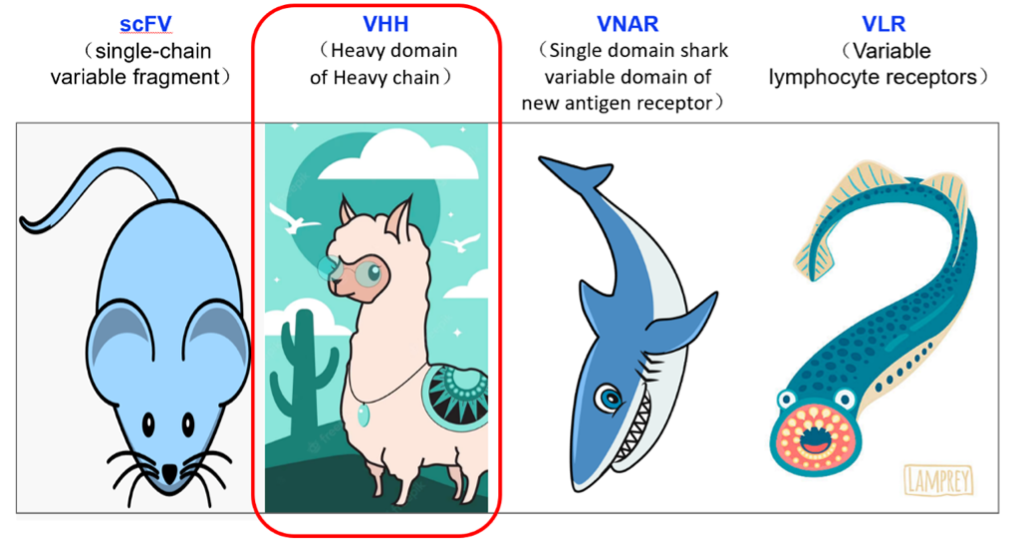
Our platform analyzed PCP scores for CAR constructs across four antibody domain types: single-chain variable fragment (scFv), camelid-derived nanobodies (VHH), shark-derived nanobodies (VNAR), and variable lymphocyte receptors derived from jawless vertebrates (VLR). Remarkably, VHH-based CARs consistently fell within the optimal range. This AI-driven approach not only streamlines the design process but also significantly accelerates the development of potent CAR-T therapies (1).
Overcoming Challenges: A Continuous Journey
CAR-Toner has significantly reduced the trial-and-error of CAR-T cell design. Yet, it is not without its limitations. While the AI provides strategies for adjusting PCP scores, it cannot fully predict the potential side effects that may emerge. Consequently, laboratory validation remains a critical step in the design process. Our goal is for AI to not only suggest multiple viable CAR-T designs but also to enable us to refine and select the most effective candidates for clinical trials.
Towards a Full-cycle AI Platform
Looking ahead, we're developing a comprehensive AI platform that could take any target antigen related to cancer therapy, anti-aging, or anti-infection and provide a ready-to-use, optimized CAR-T design. Our commitment is unwavering: to propel CAR-T therapy into the future, where AI is not just a tool but a transformative force.
References:
1. Qiu S, Chen J, Wu T, et al. CAR-Toner: An AI-driven approach for CAR tonic signaling prediction and optimization. Cell Research 2024;33(5):341-54. (CAR-Toner Website: http://cart-fitness.slst.shanghaitech.edu.cn/CAR-fitness/)
2. Li W, Qiu S, Chen J, et al. Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy. Immunity 53 (2), 456-470. e6.
3. Weber EW, Maus MV, Mackall CL. The Emerging Landscape of Immune Cell Therapies. Cell. 2020;181(1):46-62.
4. Wang H, Song X, Shen L, et al. Exploiting T cell signaling to optimize engineered T cell therapies. Trends in Cancer 8 (2), 123-134.
5. Chen J, Qiu S, Li W, et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Research 2023;33(5):341-54.
6. Wang H, Huang Y, Xu, et al. Charging CAR by electrostatic power. Immunological Reviews 320 (1), 138-146.
Follow the Topic
-
Cell Research

This journal publishes original research results that are of unusual significance or broad conceptual or technical advances in all areas of life sciences, as long as the study is closely related to molecular and cell biology.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in