The asymmetric solvating design: a versatile electrolyte design strategy for micro-sized alloying anodes
Motivation
Silicon (Si) anodes represent one of the most promising alloying materials to upgrade the current Li-ion batteries (LIBs) to next-generation high-energy batteries due to its high theoretical capacity (3579 mAh g-1 of Li15Si4 vs 372 mAh g-1 of LiC6), low electrochemical potential (~0.3 V vs Li/Li+), and natural abundance1. However, the large volume change (~300%) from Si to Li15Si4 during lithiation/delithiation cycles induces cracks of Si particles and continuous formation of unfavorable solid electrolyte interface (SEI), which results in irreversible capacity loss, low Coulombic efficiency (CE) and poor cell cycle life2. The highlighted strategies to tackle the volume expansion issue for Si anodes include downsizing the particles to nano-region or integrating the nanoSi with graphite (Gr) to form the nanoSi-Gr composite anodes3. Nevertheless, nano-sized Si particles will increase the manufacturing cost and reduce the cell calendar life, and the nanoSi-Gr composite anodes will also sacrifice the cell energy density. Therefore, from both the economic and energy utilization perspective, micro-sized Si (uSi) is the ideal material for high-energy batteries.
Electrolyte Challenges
For commercial carbonate-based electrolytes, it is very challenging to achieve efficient electrochemical reversibility on alloying anodes due to the inevitable cracks of both alloy particles and organic SEI during the repeated charge/discharge cycles (Figure 1a). A more robust SEI is required to accommodate the large stress and volume change of the alloying anodes for long-term cycle performance (Figures b-c).
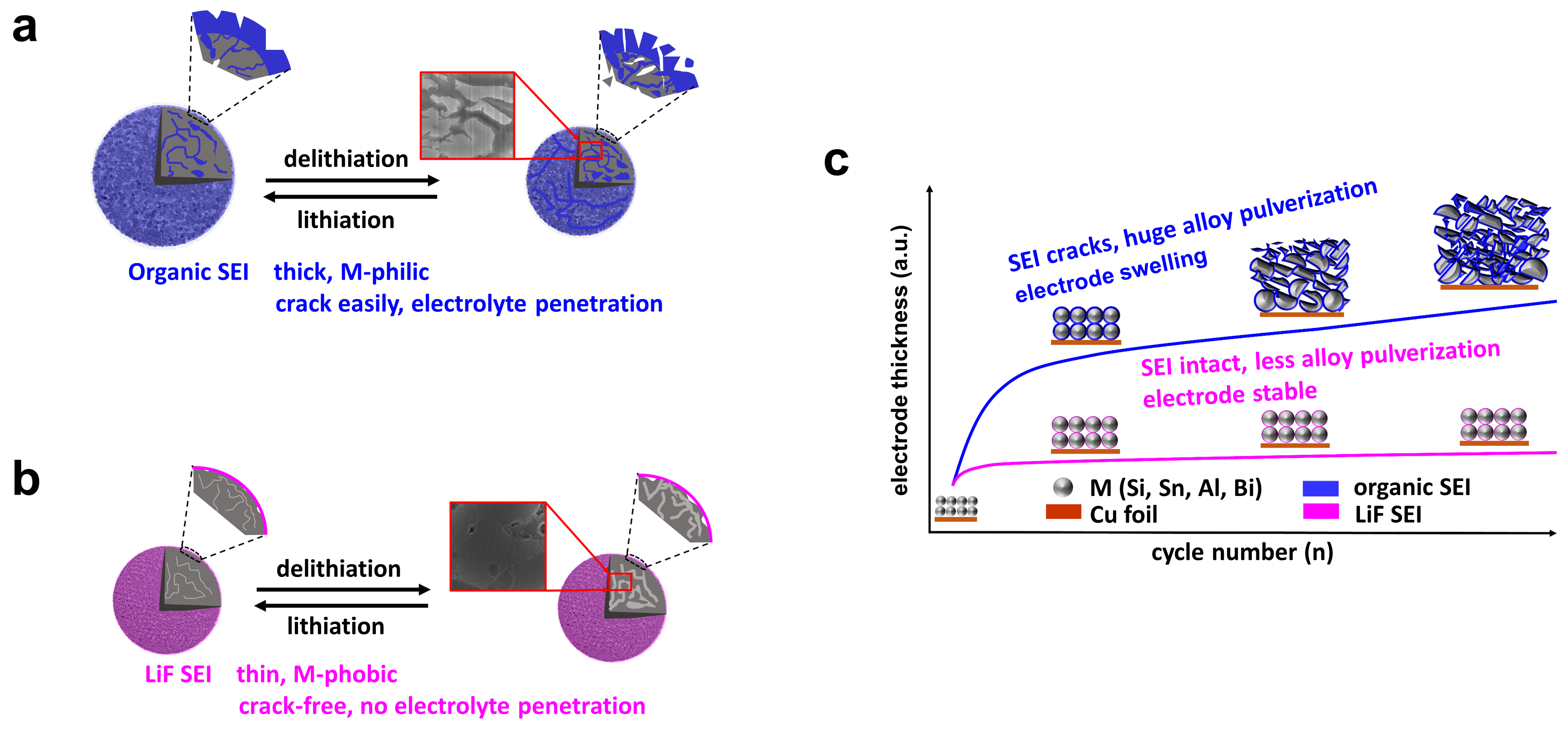
The key to realizing high reversibility on alloying anodes is to construct high-modulus SEI (e.g. LiF) that has a weak bond to the LixM phase (M-phobic) (Figure 1b), therefore reversible volume expansion/contraction of the LixM phase can be achieved inside the M-phobic SEI shell, allowing for prolonged cycle performance. Hence, the M-philic organic SEI (Figure 1a) from solvent reduction needs to be suppressed, and the M-phobic inorganic SEI from anion reduction should be promoted. Unless the reduction of the solvent can provide beneficial SEI components like Li2O4. Our previous work indicates that the PF6- anion reduction is favorable over FSI- or TFSI- anions for effective LiF SEI construction on alloying anodes when using low-reduction solvents5. However, the LiPF6 salt has been found incompatible with the widely used ether solvent like dimethoxyethane (DME) due to their easy polymerization6. The challenging question is, is there a way to make the low-reduction ether solvents (with high boiling point) compatible with LiPF6 salt?
Strategy and Key Findings
Recently, molecular design has arisen as an efficient tool to realize advanced electrolyte development and ionic liquid electrolytes have been long pursued due to their environmental friendliness and high safety. To make the DME solvent compatible with LiPF6 salt, we chemically introduced the “DME” fragment into the methylpyrrolidinium cation, forming the new ionic liquid, N-Methyl-N-(2-methoxyEthoxy)methyl Pyrrolidinium hexafluorophosphate (abbreviated as NMEP). This asymmetric molecular design resolved the long-lasting DME/LiPF6 incompatible issues and brought several beneficial advantages for NMEP/LiPF6 electrolytes to be used on alloying anodes (Figure 2).
(1) The asymmetric design with bulky methylpyrrolidinium cation on one side of the “DME” fragment stops the repeated unit of {Li+(DME)n}(PF6-), making “DME” compatible with LiPF6 salt;
(2) The ether-functionalization reduces the melting point of NMEP below room temperature, combined with the low-reduction of methylpyrrolidinium cation, the NMEP/LiPF6 electrolytes will promote the formation of LiF SEI on alloying anodes same as mTHF/THF/LiPF6 electrolytes;
(3) The ionic nature of NMEP improves the anodic stability of NMEP/LiPF6 electrolytes, therefore high-energy cathodes like NMC811 can be paired with alloying anodes to achieve high energy density.
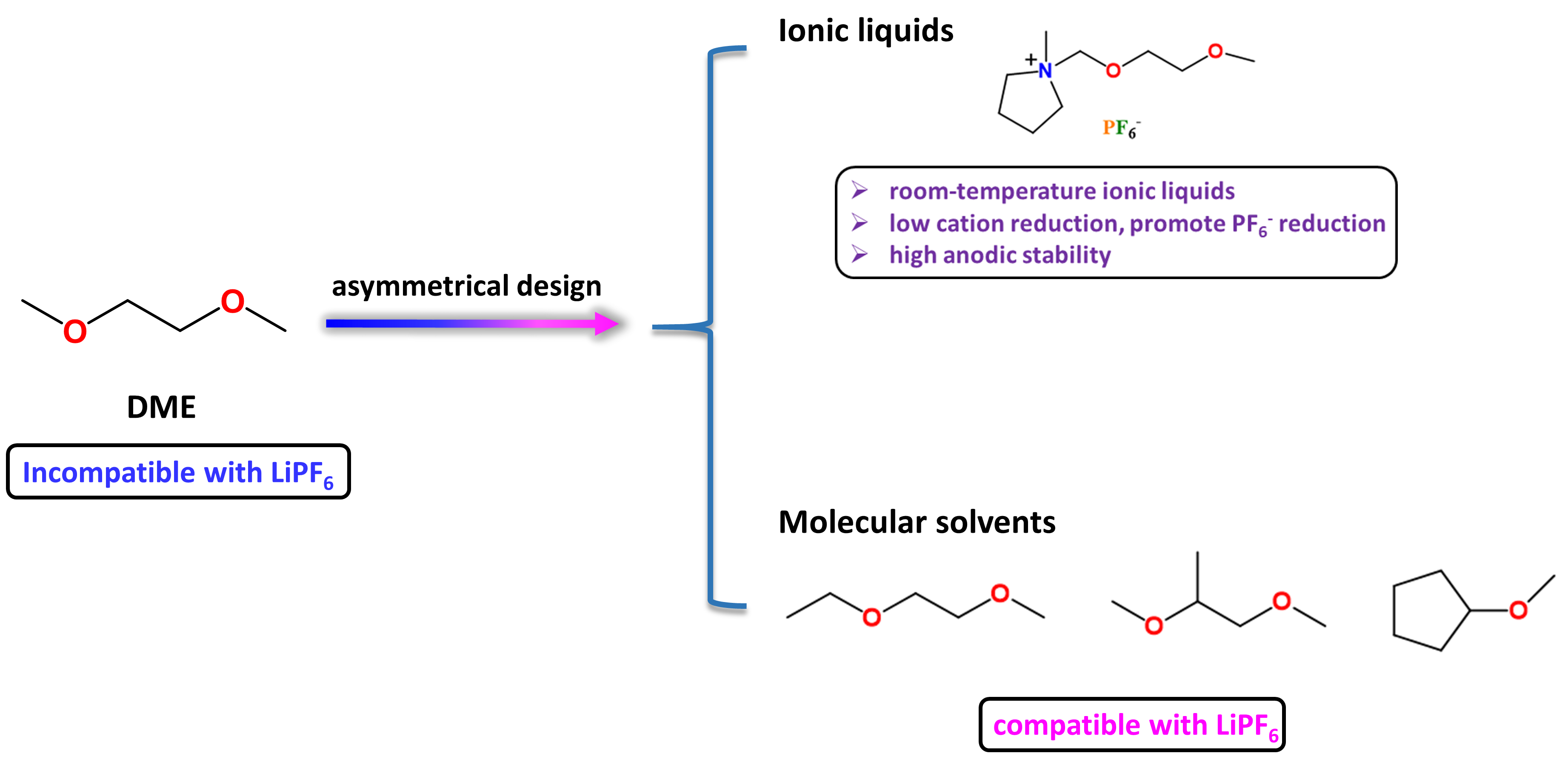
Figure 2 | The Asymmetric Molecular Design Strategy. The introduction of the “DME” fragment into 1-methyl-pyrrolidinium cation breaks the symmetry of the “DME”, facilitating the compatibility between “DME” and LiPF6 salt, therefore the formulation of NMEP/LiPF6 electrolytes. This concept also applies to molecular solvents as illustrated in 1-ethoxy-2-methoxyethane (EME), 1,2-dimethoxy propane (DMP), and cyclopentyl methyl ether (CPME), making the asymmetric solvating design unique for ether/LiPF6 electrolytes on micro-sized alloying anodes.
As a result, the developed NMEP/LiPF6 electrolytes enabled uSi anodes (325 mesh, Sigma) to deliver a high capacity of >2800 mAh g-1 for 400 cycles with a capacity retention of >80% and cycle CE of >99.9%. Other low-cost alloying anodes like Al, Sn, Bi also showed excellent cycle performance in the proposed electrolytes, validating its universal application. The uSi||NMC811 full cells were demonstrated for over 200 cycles with a capacity retention of >85%, showing great promise for high-energy batteries. However, the ionic liquid electrolytes are viscous and have issues at higher cycle rates. Inspired by the asymmetric cation design in the NMEP/LiPF6 electrolytes, we further evaluated the compatibility between LiPF6 salt and other asymmetric ether solvents. As shown in Figure 2, all three ether solvents showed good solubility for LiPF6 salt (>2.0 M) without polymerization. We demonstrated that the EME/LiPF6 electrolytes enable the uSi anodes (325 mesh, Sigma) to deliver >1100 mAh g-1 capacity at 5C rate, and the uSi||NMC811 full cells for 200 cycles at C/3 rate with a high capacity retention of >90%, projecting high commercialization potential for uSi||NMC811 batteries.
Implications
As the development of electrolytes for uSi anodes is currently still at an early stage, the asymmetric design in the present work opens new doors for engineering advanced electrolytes using ether solvents and LiPF6 salt, which has been thought impossible before. We believe that the formulation between the asymmetric ether solvents and LiPF6 salt is expected to become the preferred industrial electrolyte for uSi anodes. Another important message of this work is that the solvation structure of the ether/LiPF6 electrolytes is also vital for effective LiF SEI formation, which can be fine-tuned for state-of-the-art electrolytes.
More details of this work can be found in our article published in Nature Energy
Ai-Min Li, …et al. Chunsheng Wang* Asymmetric Electrolyte Design for High-Energy Lithium-Ion Batteries with Micro-sized Alloying Anodes. Nat. Energy (2024).
DOI: https://doi.org/10.1038/s41560-024-01619-2
Contributors: Ai-Min Li, Professor Chunsheng Wang
Reference
- Obrovac, M. N. et al. Alloy Design for Lithium-Ion Battery Anodes. J. Electrochem. Soc. 154, A849 (2007).
- Liu, X. H. et al. Anisotropic swelling and fracture of silicon nanowires during lithiation. Nano Lett. 11, 3312–3318 (2011).
- Sung, J. et al. Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack. Nat. Energy 6, 1164-1175 (2021).
- Li, A. M. et al. High voltage electrolytes for lithium-ion batteries with micro-sized silicon anodes. Nat. Commun. 15, 1206 (2024).
- Chen, J. et al. Electrolyte design for LiF-rich solid–electrolyte interfaces to enable high-performance microsized alloy anodes for batteries. Nat. Energy 5, 386-397 (2020).
- Mac Glashan, G. S. et al. Structure of the polymer electrolyte poly (ethylene oxide)6: LiAsF6. Nature 398, 792-794 (1999).
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in