The bifurcation point of arterial and hemogenic fate determination
Published in Cell & Molecular Biology
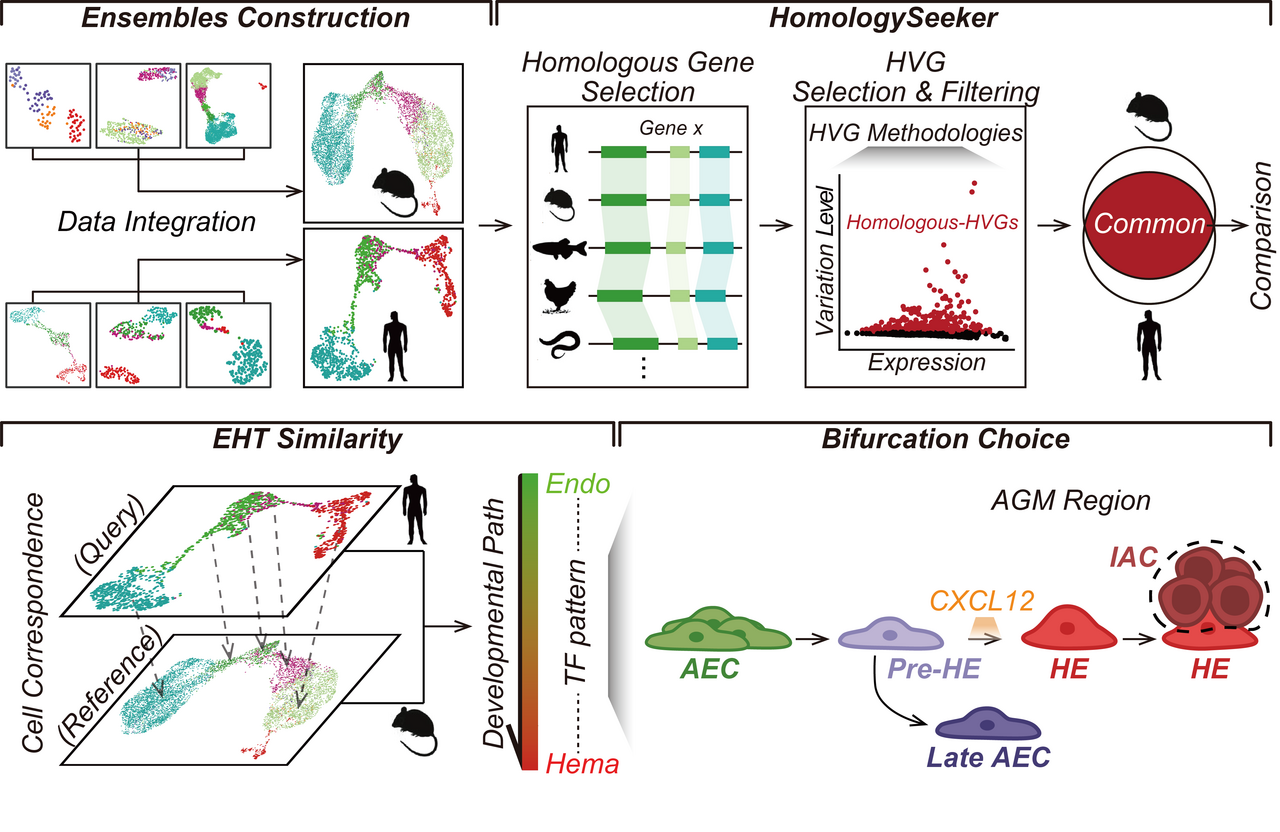
Embryonic development is a rich tapestry of cell fate decisions, where each cell makes choices that shape the formation of our bodies. One intriguing choice occurs when certain cells decide to become either artery cells or blood cells. This post delves into the process where our first blood stem cells are born - a journey that starts from a group of cells known as 'hemogenic endothelium'. This process is not only crucial for our understanding of normal development but also offers rich insights into our understanding of blood diseases such as leukemia.
We know from studies in mouse that these blood-forming cells come from early arterial cells, but why some choose the blood route and others stick to the arterial path is a captivating mystery. Our interest was piqued by cells in a stage we've called 'pre-hemogenic endothelium,' which appear to be at the threshold of this critical decision. The question is - how do these cells choose their destiny, and what factors tip the scales in favor of one decision over the other?
This is where our computational tool, 'HomologySeeker,' steps in. Acting as a translator between the 'languages' of mouse and human genes, HomologySeeker enabled us to identify genes that function similarly across species and potentially hold the keys to understanding this cell fate decision. In our study, we analyzed an extensive collection of single-cell transcriptome data from the mouse and human endothelial-to-hematopoietic transition (EHT) process. This data was carefully integrated to form EHT transcriptome ensembles, which served as our basis for comparison. Through a customized analysis pipeline from our HomologySeeker algorithm and other bioinformatic tools, we have established a developmental relationship between mouse and human, a parallel that proves intriguing.
One particularly interesting discovery is the faint trace of hematopoietic stem cell precursor (pre-HSC) signals in human cells. In the mouse model, T1 and T2 pre-HSCs serve as a direct precursor of HSC, a critical role in the EHT process. T1 pre-HSCs demonstrate hematopoietic potential following a hematopoietic culture, while T2 pre-HSCs do not require additional cultivation. These pre-HSCs carry unique surface markers that set them apart from other cells participating in the EHT process. Consequently, if a corresponding group of pre-HSCs could be successfully isolated in humans, it opens up the fascinating possibility of large-scale in vitro expansion. By utilizing precise markers, we could then select a subgroup that possesses HSC potential, thereby improving the efficiency of the ex vivo expansion of HSCs. This is indeed an exciting direction in our research.
Further, we identified a unique cell group in human, termed C5 in the manuscript, which uniquely exhibits both arterial and hemogenic characteristics. What's interesting is that this group does not seem to participate in subsequent EHT processes. This finding underscores the diversity in the fate choice of early arterial endothelial Cells (AECs), suggesting there are options beyond the traditional paths towards hemogenic endothelium (HE) and late AECs. This opens up new avenues for exploration and understanding the underlying complexity of EHT.
Central to our research was the discovery of the critical role the pre-hemogenic endothelium (pre-HE) stage plays. This stage acts as a fork in the road, where cells decide to either become blood cells or continue as arterial cells. This finding raises the following questions: what makes pre-HE so special? Specifically, what mechanisms allow cells at the pre-HE stage to choose different fates? How does Runx1 precisely regulate the hemogenic fate decision of pre-HE? Whether the fate determination of pre-HEs toward late AEC is influenced by their inability to bypass the developmental bottleneck? Or might different AEC subpopulations already have their fates predetermined? In total, dissecting the above questions will help us to gain a deeper understanding of the critical events involved in the hemogenic fate choice.
Our exploration led to the identification of a molecule, CXCL12, which might play a critical role in steering cells toward the hematopoietic potential of hemogenic endothelium. This finding is particularly surprising as previous research primarily associated CXCL12 with maintaining the quiescent HSC pool. This discovery opens up a new perspective on the function of CXCL12 and its potential role in the human EHT process. Yet, it's important to note that the exact mechanisms by which CXCL12 influences hemogenic fate determination still require thorough investigation. Intriguingly, these findings raise the possibility that the hematopoietic potential might already be predetermined during the hemogenic stage.
Our findings offer a closer view of the intricate process of human blood cell formation. Considering the close relationship between definitive HSC and arterial features, understanding the mechanisms involved in the transition of arterial cells toward hemogenic fate might offer valuable insights. This could prove crucial for creating more efficient strategies for generating HSCs in ex vivo systems, ultimately leading to advancements in stem cell therapy and regenerative medicine.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in