The division between T cell and B cell might not always be obvious
Published in Immunology
The development of conventional T and B lymphocytes is fine and strictly tuned1. The phenotypical differences between T and B cells are usually thought to be clear, which can be readily discerned through detecting cell surface markers such as CD19, CD20, CD3, CD4, CD8, BCR and TCR. However, accumulating evidence indicates that the boundary between T and B cells could be ambiguous under both pathological and physiological circumstances.
T cell/B cell mixed phenotypic lymphocytes, such as CD3+CD20+2,3 and CD3+CD19+ cells4,5 have been observed in different disease contexts3,6,7 and these biphenotypic cells are believed to play functional roles in multiple diseases, such as multiple sclerosis8, HIV infection9, rheumatoid arthritis10, ovarian cancer11 and type I diabetes4. However, their presence and function in physiological conditions remain poorly understood. And Despite of being intensively observed, the origin of these biphenotypic lymphocytes is controversial2,8,11-15.
In this study, we reported that a subset of lymphocytes endogenously expressing both T- and B-cell lineage markers (CD3, CD19, TCR-β, IgM, etc.) was identified in mice by the methods of multi-parameter flow cytometry and single-cell RNA sequencing and (Figure 1A and 1B). These CD3+CD19+ biphenotypic lymphocytes distributed widely in mouse bone marrow, lymph nodes, spleen, and peripheral blood and showed an origin of pro/pre B cells in pseudo-time analysis (Figure 1C). Furthermore, we found that these CD3+CD19+cells can be activated through both the TCR and BCR signaling pathways, and they actively participated in both cellular (Figure 1D) and humoral immune responses (Figure 1E) elicited by a candidate SARS-CoV-2 DNA vaccine. Interestingly, compared to conventional T cells (CD3+CD19- cells), CD3+CD19+ cells secreted a higher level of IL-2 but a lower level of TNF-α upon antigen-specific stimulation (Figure 1D).
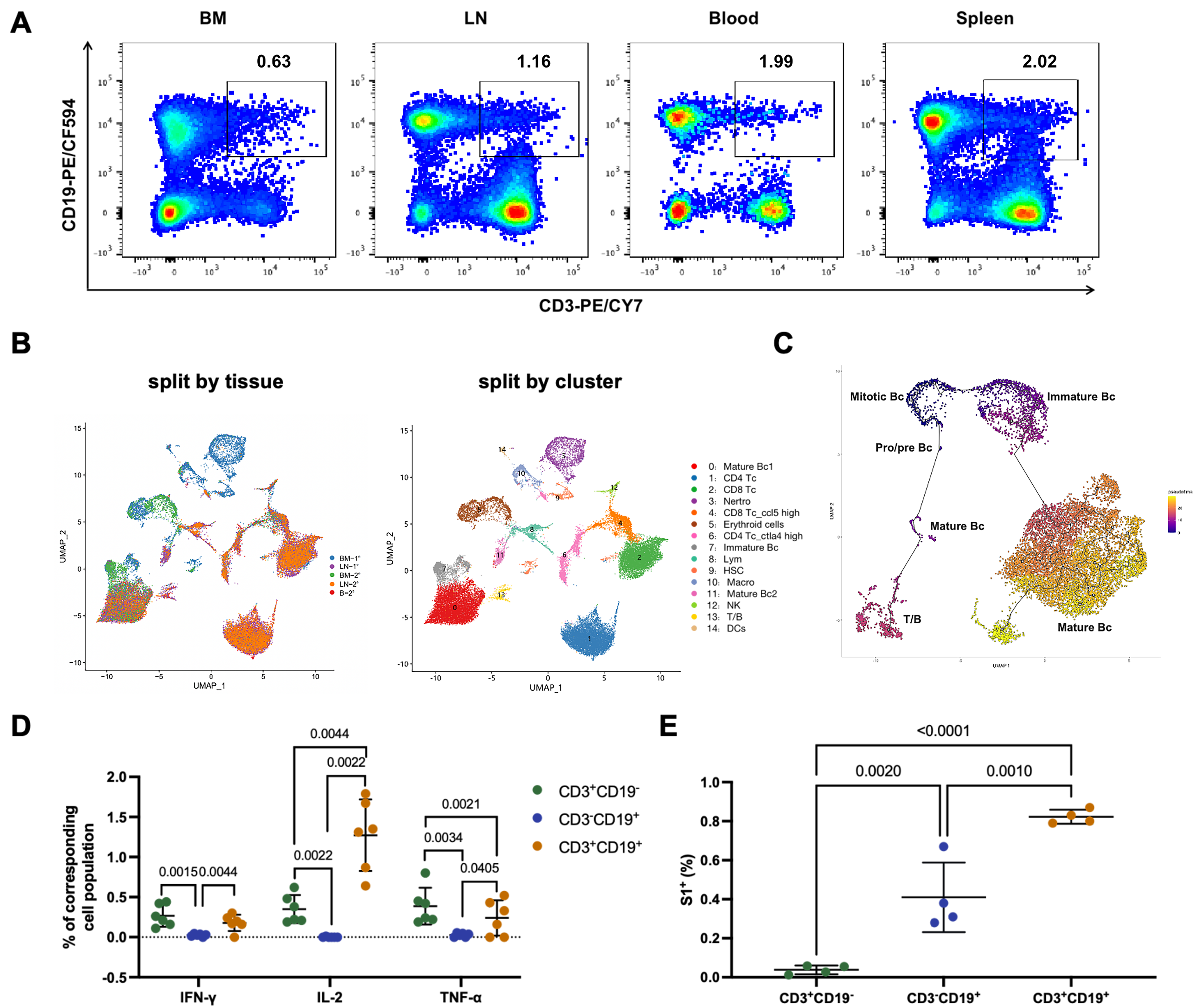
Figure 1. Functional T cell/B cell mixed phenotypic lymphocytes (CD3+CD19+) in mice.
In addition to mice, we also identified CD3+CD19+ lymphocytes in freshly isolated human peripheral blood mononuclear cells. Notably, the human CD3+CD19+ lymphocyte subset exhibited comparable functionality to its murine counterpart, albeit with a lower frequency in humans than in mice.
Building upon these findings, our findings offered a fresh perspective on the complexity of the immune system, and highlighted that the T/B mixed phenotypic lymphocytes might played unrecognized roles in adaptive immunity. Further investigations are warranted to elucidate the precise mechanisms governing the development and the functionality of these biphenotypic lymphocytes, as well as their potential contribution to immune responses in different physiological and pathological contexts.
Reference
1. Montecino-Rodriguez, E. & Dorshkind, K. The layered development of mouse B and T Cells. Immunol Rev, doi:10.1111/imr.13181 (2022).
2. Schuh, E. et al. Features of Human CD3+CD20+ T Cells. J Immunol 197, 1111-1117, doi:10.4049/jimmunol.1600089 (2016).
3. Chen, Q., Yuan, S., Sun, H. & Peng, L. CD3(+)CD20(+) T cells and their roles in human diseases. Hum Immunol 80, 191-194, doi:10.1016/j.humimm.2019.01.001 (2019).
4. Ahmed, R. et al. A Public BCR Present in a Unique Dual-Receptor-Expressing Lymphocyte from Type 1 Diabetes Patients Encodes a Potent T Cell Autoantigen. Cell 177, 1583-1599.e1516, doi:10.1016/j.cell.2019.05.007 (2019).
5. Li, Q., Wang, J., Zhang, M., Tang, Y. & Lu, H. Discovery of CD3(+) CD19(+) cells, a novel lymphocyte subset with a potential role in human immunodeficiency virus-Mycobacterium tuberculosis coinfection, using mass cytometry. Clin Transl Med 11, e681, doi:10.1002/ctm2.681 (2021).
6. Mangogna, A. et al. Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma Not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature. Diagnostics (Basel) 10, doi:10.3390/diagnostics10060341 (2020).
7. Tsuyama, N. et al. Clinical and prognostic significance of aberrant T-cell marker expression in 225 cases of de novo diffuse large B-cell lymphoma and 276 cases of other B-cell lymphomas. Oncotarget 8, 33487-33500, doi:10.18632/oncotarget.16532 (2017).
8. von Essen, M. R. et al. Proinflammatory CD20+ T cells in the pathogenesis of multiple sclerosis. Brain 142, 120-132, doi:10.1093/brain/awy301 (2019).
9. Förster, F., Singla, A., Arora, S. K., Schmidt, R. E. & Jacobs, R. CD20+ T cell numbers are decreased in untreated HIV-1 patients and recover after HAART. Immunol Lett 146, 74-78, doi:10.1016/j.imlet.2012.05.004 (2012).
10. Wilk, E. et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum 60, 3563-3571, doi:10.1002/art.24998 (2009).
11. de Bruyn, M. et al. CD20(+) T cells have a predominantly Tc1 effector memory phenotype and are expanded in the ascites of patients with ovarian cancer. Oncoimmunology 4, e999536, doi:10.1080/2162402x.2014.999536 (2015).
12. Vlaming, M. et al. CD20 positive CD8 T cells are a unique and transcriptionally-distinct subset of T cells with distinct transmigration properties. Sci Rep 11, 20499, doi:10.1038/s41598-021-00007-0 (2021).
13. Murayama, Y. et al. Transient expression of CD20 antigen (pan B cell marker) in activated lymph node T cells. Microbiol Immunol 40, 467-471, doi:10.1111/j.1348-0421.1996.tb01096.x (1996).
14. Quintanilla-Martinez, L., Preffer, F., Rubin, D., Ferry, J. A. & Harris, N. L. CD20+ T-cell lymphoma. Neoplastic transformation of a normal T-cell subset. Am J Clin Pathol 102, 483-489, doi:10.1093/ajcp/102.4.483 (1994).
15. Takami, A. et al. CD20-positive T-cell chronic lymphocytic leukaemia. Br J Haematol 102, 1327-1329 (1998).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in