The Efficient Extraction Method for Uranium from Nuclear Wastewater
Published in Earth & Environment

We are one of the local labs that primarily focus on the uranium resource utilization, located within State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science & Technology, in Mianyang, China. We are delighted to share the stories behind the paper while also provide fresh insights for electrochemical uranium extraction.
Uranium extraction in nuclear wastewater from the production of nuclear fuel is an efficient means to realize the secondary supply of uranium resources. In practical terms, the majority of uranium-containing nuclear wastewater is generated through fuel production processes which involve extensive use of uranium fluoride. In real nuclear wastewater, F− commonly co-exists with UO22+, resulting in the complex species of UO2Fx and thus the decreased extraction efficiency. Accordingly, the traditional adsorption or ion exchange strategy requires the pre-precipitation of F− by Ca2+, resulting in the formation of uranium-containing CaF2 as radioactive solid waste.
Parallel to traditional strategy, electrochemical uranium extraction has attracted increasing attention because of the fast kinetics, increased extraction capacity, and resistance to the interference of anions. However, current progress on electrochemical extraction of U(VI) is limited to the aqueous solution systems without F−, which lack the strategy in real nuclear wastewater from nuclear fuel production. In real nuclear wastewater, the main bottlenecks of uranium extraction lie in the competitive coordination between U(VI) and F−, thus resulting in the poor extraction efficiency of uranium under the interference of high concentrations of F−.
Therefore, we developed an ion pair sites strategy of for selective binding of UO2Fx and efficient electrochemical uranium extraction in real nuclear wastewater. The UO2Fx was adsorbed on the ion pair sites of without the separation of the U-F bond. After that, the uranyl was reduced to low-valent species, which weakened the Coulombian force of uranyl and F−, thus resulting in the formation of an intermediate UO2+x product. The metastable low-valent uranium was then oxidized, followed by crystallizing with F− and forming the final K3UO2F5 (Figure 1). In real nuclear wastewater, the uranium was extracted as oxide powder with high purity. Therefore, the uranium was able to be efficiently extracted from the electrode.
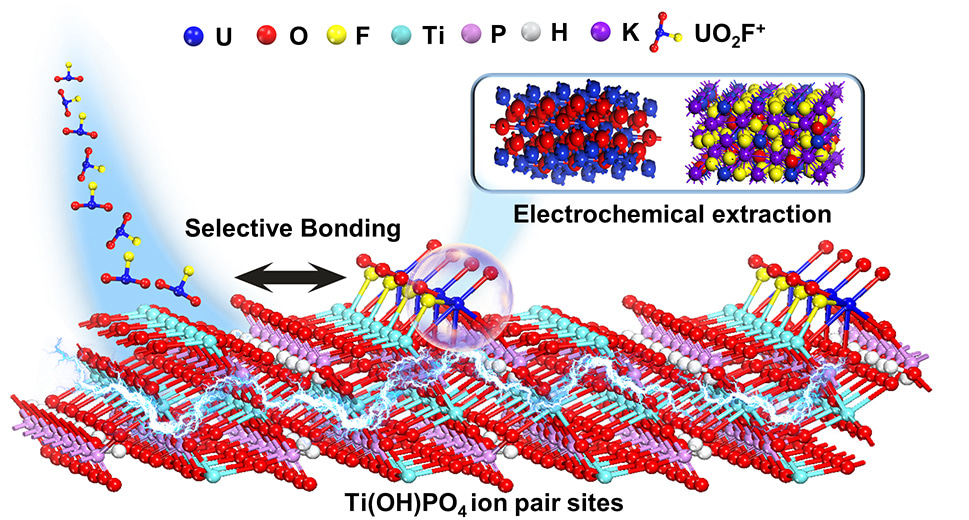
We hope that our strategy of ion pair sites in the electrochemical extraction of nuclear wastewater can serve as a beacon of inspiration for other researchers in the field. By revealing the potential applications of ion pair sites in the field of electrochemical uranium extraction, we will be more than delighted to witness further exploration into innovative coordination sites strategies for not only uranium extraction but also other complex nuclide.
Please feel free to contact Prof. Wenkun Zhu, corresponding author of this article, if you have any questions or comments about the work: zhuwenkun@swust.edu.cn.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in