The establishment of a novel HB vaccine by using the yeast-derived large-HBs antigen.
Published in Microbiology
Hepatitis B virus (HBV) infection is a major cause of chronic liver disease. In 2019, WHO estimated that 296 million people were living with chronic hepatitis B with 1.5 million new infections each year. HBV is transmitted via blood or other body fluids, and the infection occurs through sexual contact or by sharing needles or syringes, or it can be passed from mother to infant at birth. In most cases of mother-to-infant infection or infection in childhood, people cannot eliminate the infection and establish a chronic infection. Chronic HBV infection can lead to serious liver diseases, including cirrhosis and hepatocellular carcinoma.
To prevent HBV infection, the HB vaccine is available. It is considered to be the most effective approach to control the spread of HBV and reduce HBV-related morbidity and mortality. The HB vaccine comprising the small-HBs (S-HBs) antigen is inoculated worldwide and plays a major role in controlling the spread of hepatitis B infection.
This HB vaccine is known to have a strong ability to induce neutralizing antibodies with a tolerable safety profile. However, several concerns that cannot be neglected have been noted. Approximately 10% of vaccinated adults have a low or no humoral response to these vaccines. Some amino acid polymorphisms in the antigenic region (a determinant) of S-HBs are known to lead to the evasion of the virus from neutralization by the antibodies induced by HB vaccines and HBV with such polymorphisms infect even vaccinated individuals. Thus, such polymorphisms are designated vaccine-escape mutations (VEMs).
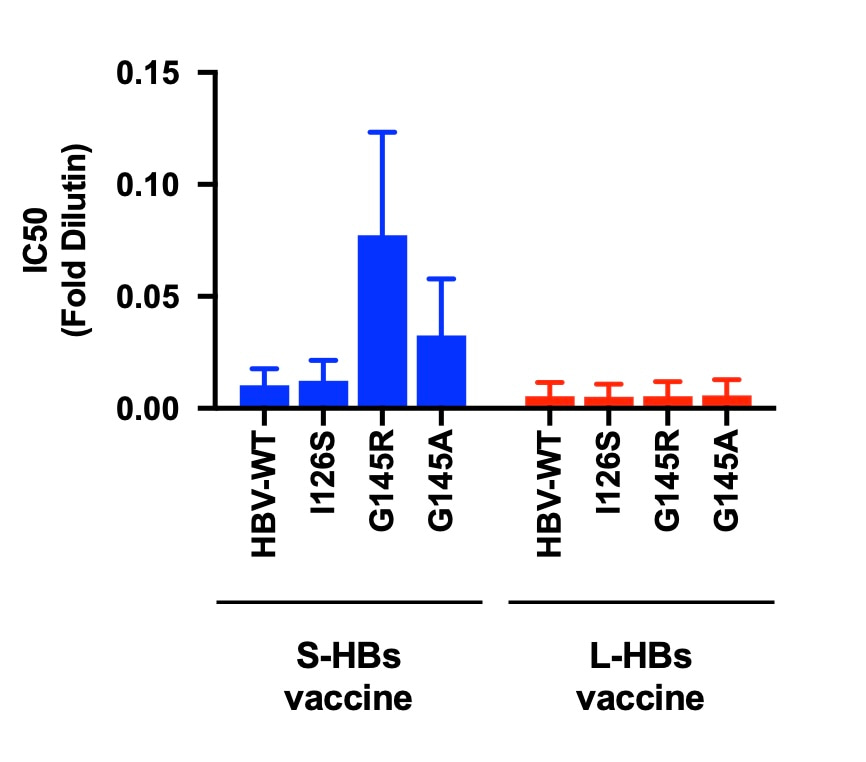
We evaluated the effects of VEMs on the neutralizing effects of HB vaccine-induced antibodies by using the nonhuman primate model. We quantified the neutralization of HBV infection by induced antibodies in serum samples of HB vaccine-immunized rhesus macaques in the infection system of HBV reporter viruses in cell culture. The representative VEMs of isoleucine to serine at amino acid 126 (I126S), glycine to arginine at amino acid 145 (G145R), and glycine to alanine at amino acid 145 (G145A) were evaluated. The VEMs of G145R and G145A certainly reduced the neutralization effects of the current HB vaccine-induced antibodies (Figure 1). To overcome this non-negligible drawback, we established a novel HB vaccine using the yeast-derived large-HBs (L-HBs) antigen. The L-HBs antigen contains the receptor binding region that is not contained in the S-HBs antigen and induces the antibodies targeting this region. It was found that the L-HBs vaccine-induced antibodies are effective against not only wild-type HBV but also HBV with VEMs. The novel HB vaccine with L-HBs will be able to compensate for the drawbacks of the current HB vaccine and be used as a supportive vaccine to the current HB vaccine.
Reference:
Washizaki, A., Murayama, A., Murata, M., Kiyohara, T., Yato, K., Yamada, N., Aly, H.H., Tanaka, T., Moriishi, K., Nishitsuji, H., Shimotohno, K., Goh, Y., Ishii, K.J., Yotsuyanagi, H., Muramatsu, M., Ishii, K., Takahashi, Y., Suzuki, R., Akari, H., and Kato, T. (2022) ‘Neutralization of hepatitis B virus with vaccine-escape mutations by hepatitis B vaccine with large-HBs antigen.’ Nature Communications. https://rdcu.be/cUXyn
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in