The importance of context in analyzing antibiotic resistance
Published in Microbiology

Our paper recently published in Microbiome is the third of what I call my trilogy on the impact of antibiotics upon the microbiome. In the first article (The ISME Journal, 2016), we determined how the intake of a commonly used antibiotic modulated the fecal microbiome. One of the strengths of this study was that we used deep shotgun metagenomics (up to 15 Gb per sample), which allowed us to not only do taxonomical profiling of the microbiomes, but also to mine these metagenomes for antibiotic resistance genes. We detected more than 43 000 putative antibiotic resistance genes!
Yes, 43 000 is a really big number. That’s a lot of potentially antibiotic resistant bacteria in only 24 healthy individuals (at three time points). At first, I was worried. Today, I see this number from a wholly different perspective.
A few months after the publication of this first article (and of the second also), I had the pleasure to attend the Advanced course on antibiotics organized by the Fondation Mérieux and the Institut Pasteur at Les Pensières, in Annecy, France (see picture above). In a nutshell, this was a two-week course about antibiotics and antibiotic resistance, given by more than a dozen experts in different areas related to the topic. It was more than a course, but also a human experience. At some point in the training, I was invited to briefly present about antibiotic resistance and the microbiome. I showed unpublished figures related to our 2016 ISME paper where specific antibiotic resistance genes were listed as prevalent in our microbiomes. At this point, an eminent microbiologist raised his hand and commented: “Many of these are present in all strains of E. coli. They are not clinically relevant. They are everywhere!”
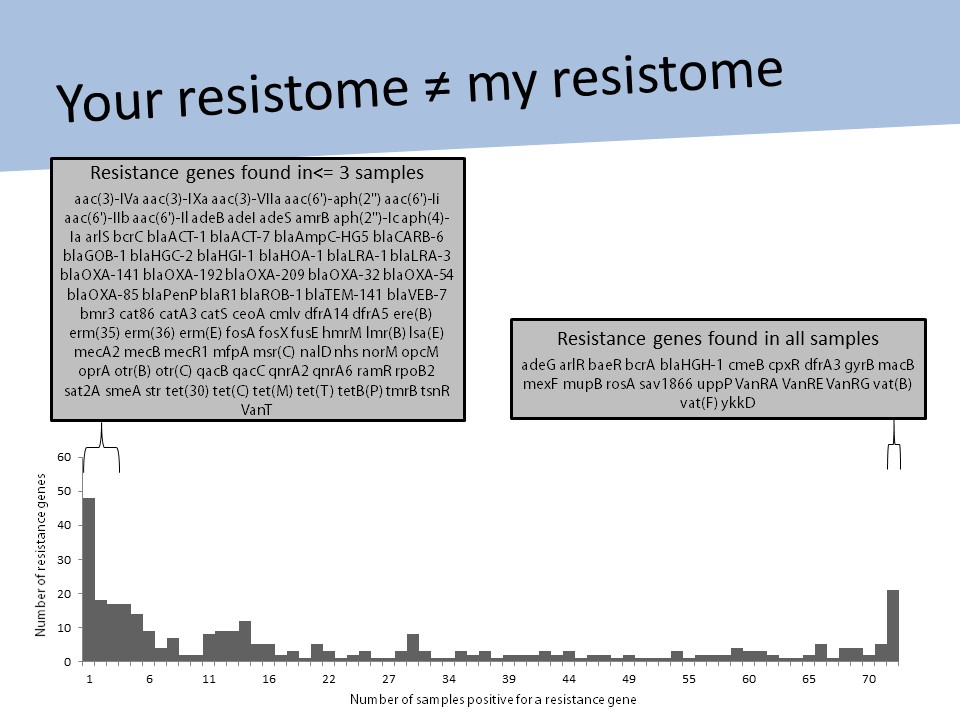
This got me thinking and, gradually, I became aware that these putative antibiotic resistance genes needed much more than cataloguing. 43 000 was just a scary number, it didn’t mean anything. These genes needed context.
Genomic context.
Epidemiological context.
Clinical context.
In the following year, my colleagues and I investigated approaches to conduct comparative genomics from a big data perspective (for example by using our Ray Surveyor software). This led me to wonder how we could use this expertise to better interpret the gene content of microbiomes based on genomes available in public databases. This is how we proceeded:
- Performed taxonomical identification of contigs containing antibiotic resistance genes.
- Annotated antibiotic resistance genes in all the available genomes from a bacterial species (for example E. coli).
- Compared the antibiotic resistance genes found in contigs potentially originating from E. coli to the genes that were actually found in E. coli genomes.
I won’t repeat here what we observed from these analyses (if you are interested, the paper is here), but it did help us in determining which antibiotic resistance genes were potentially relevant, and which had the most potential for horizontal gene transfer. How did we do that? By considering the genomic environment of these genes along with their distribution in different isolates.
The take-home message here is a single word: context.
There are so many sequences available to help contextualize our data, that we would be crazy not to use them. Truly, the only limitation here is our imagination. And maybe our computing power…
For more details, see our manuscript here: https://microbiomejournal.biomedcentra.com/articles/10.1186/s40168-019-0669-7
Follow the Topic
-
Microbiome

This journal hopes to integrate researchers with common scientific objectives across a broad cross-section of sub-disciplines within microbial ecology. It covers studies of microbiomes colonizing humans, animals, plants or the environment, both built and natural or manipulated, as in agriculture.
Related Collections
With Collections, you can get published faster and increase your visibility.
Harnessing plant microbiomes to improve performance and mechanistic understanding
This is a Cross-Journal Collection with Microbiome, Environmental Microbiome, npj Science of Plants, and npj Biofilms and Microbiomes. Please click here to see the collection page for npj Science of Plants and npj Biofilms and Microbiomes.
Modern agriculture needs to sustainably increase crop productivity while preserving ecosystem health. As soil degradation, climate variability, and diminishing input efficiency continue to threaten agricultural outputs, there is a pressing need to enhance plant performance through ecologically-sound strategies. In this context, plant-associated microbiomes represent a powerful, yet underexploited, resource to improve plant vigor, nutrient acquisition, stress resilience, and overall productivity.
The plant microbiome—comprising bacteria, fungi, and other microorganisms inhabiting the rhizosphere, endosphere, and phyllosphere—plays a fundamental role in shaping plant physiology and development. Increasing evidence demonstrates that beneficial microbes mediate key processes such as nutrient solubilization and uptake, hormonal regulation, photosynthetic efficiency, and systemic resistance to (a)biotic stresses. However, to fully harness these capabilities, a mechanistic understanding of the molecular dialogues and functional traits underpinning plant-microbe interactions is essential.
Recent advances in multi-omics technologies, synthetic biology, and high-throughput functional screening have accelerated our ability to dissect these interactions at molecular, cellular, and system levels. Yet, significant challenges remain in translating these mechanistic insights into robust microbiome-based applications for agriculture. Core knowledge gaps include identifying microbial functions that are conserved across environments and hosts, understanding the signaling networks and metabolic exchanges between partners, and predicting microbiome assembly and stability under field conditions.
This Research Topic welcomes Original Research, Reviews, Perspectives, and Meta-analyses that delve into the functional and mechanistic basis of plant-microbiome interactions. We are particularly interested in contributions that integrate molecular microbiology, systems biology, plant physiology, and computational modeling to unravel the mechanisms by which microbial communities enhance plant performance and/or mechanisms employed by plant hosts to assemble beneficial microbiomes. Studies ranging from controlled experimental systems to applied field trials are encouraged, especially those aiming to bridge the gap between fundamental understanding and translational outcomes such as microbial consortia, engineered strains, or microbiome-informed management practices.
Ultimately, this collection aims to advance our ability to rationally design and apply microbiome-based strategies by deepening our mechanistic insight into how plants select beneficial microbiomes and in turn how microbes shape plant health and productivity.
This collection is open for submissions from all authors on the condition that the manuscript falls within both the scope of the collection and the journal it is submitted to.
All submissions in this collection undergo the relevant journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief of the relevant journal. As an open access publication, participating journals levy an article processing fee (Microbiome, Environmental Microbiome). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief of the journal where the article is being submitted.
Collection policies for Microbiome and Environmental Microbiome:
Please refer to this page. Please only submit to one journal, but note authors have the option to transfer to another participating journal following the editors’ recommendation.
Collection policies for npj Science of Plants and npj Biofilms and Microbiomes:
Please refer to npj's Collection policies page for full details.
Publishing Model: Open Access
Deadline: Jun 01, 2026
Microbiome and Reproductive Health
Microbiome is calling for submissions to our Collection on Microbiome and Reproductive Health.
Our understanding of the intricate relationship between the microbiome and reproductive health holds profound translational implications for fertility, pregnancy, and reproductive disorders. To truly advance this field, it is essential to move beyond descriptive and associative studies and focus on mechanistic research that uncovers the functional underpinnings of the host–microbiome interface. Such studies can reveal how microbial communities influence reproductive physiology, including hormonal regulation, immune responses, and overall reproductive health.
Recent advances have highlighted the role of specific bacterial populations in both male and female fertility, as well as their impact on pregnancy outcomes. For example, the vaginal microbiome has been linked to preterm birth, while emerging evidence suggests that gut microbiota may modulate reproductive hormone levels. These insights underscore the need for research that explores how and why these microbial influences occur.
Looking ahead, the potential for breakthroughs is immense. Mechanistic studies have the power to drive the development of microbiome-based therapies that address infertility, improve pregnancy outcomes, and reduce the risk of reproductive diseases. Incorporating microbiome analysis into reproductive health assessments could transform clinical practice and, by deepening our understanding of host–microbiome mechanisms, lay the groundwork for personalized medicine in gynecology and obstetrics.
We invite researchers to contribute to this Special Collection on Microbiome and Reproductive Health. Submissions should emphasize functional and mechanistic insights into the host–microbiome relationship. Topics of interest include, but are not limited to:
- Microbiome and infertility
- Vaginal microbiome and pregnancy outcomes
- Gut microbiota and reproductive hormones
- Microbial influences on menstrual health
- Live biotherapeutics and reproductive health interventions
- Microbiome alterations as drivers of reproductive disorders
- Environmental factors shaping the microbiome
- Intergenerational microbiome transmission
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 16, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in