The journey to SPA for volumetric structured illumination microscopy
Published in Chemistry, Physics, and Protocols & Methods
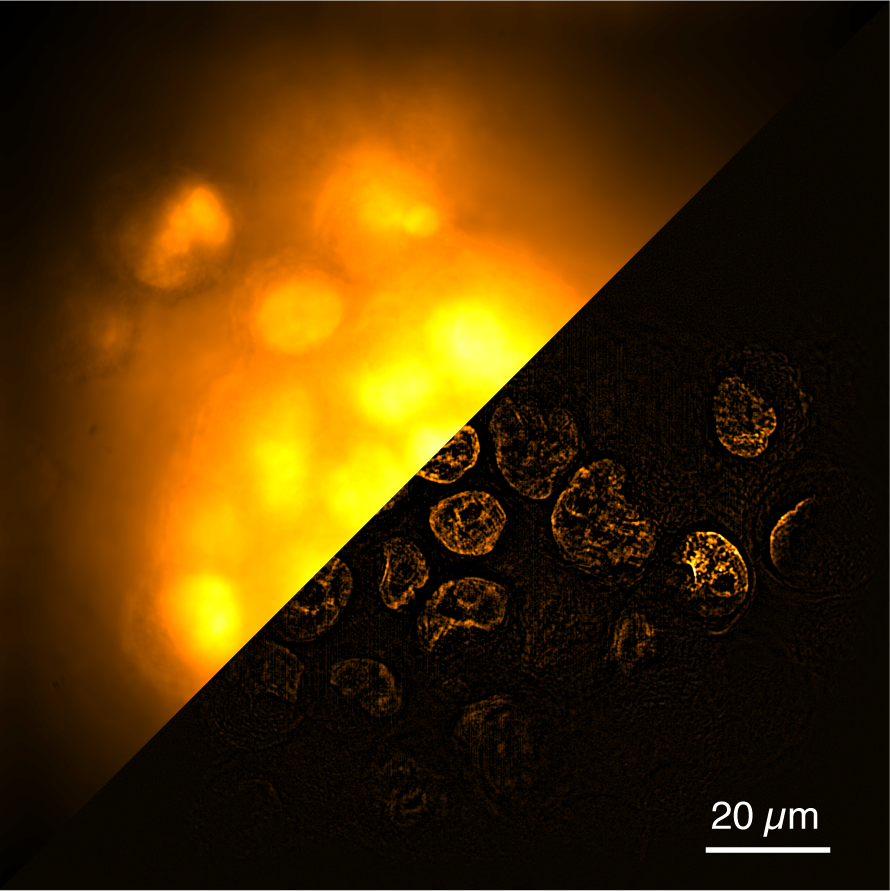
The concept of SPA-SIM integrates two distinct illumination patterns using reversibly photoswitchable fluorescent proteins (RSFPs). We employed selective plane illumination for depth discrimination during the activation of RSFPs and structured illumination for super-resolution during excitation. This combination resulted in a thin stripe pattern as the fluorescence emission for 3D super-resolution imaging. SPA-SIM effectively suppresses out-of-focus light, resolving the fundamental artifact issue in SIM and enabling 3D imaging of thick and dense objects.
To bring this concept to life, careful consideration was required for mounting the SPA-SIM components on an optical table. For instance, the two objectives needed to be positioned orthogonally above the sample holder to provide side illumination for selective plane activation and epi-illumination for structured illumination. Additionally, several optical elements had to be similarly arranged to deliver the illumination beams to these objectives.
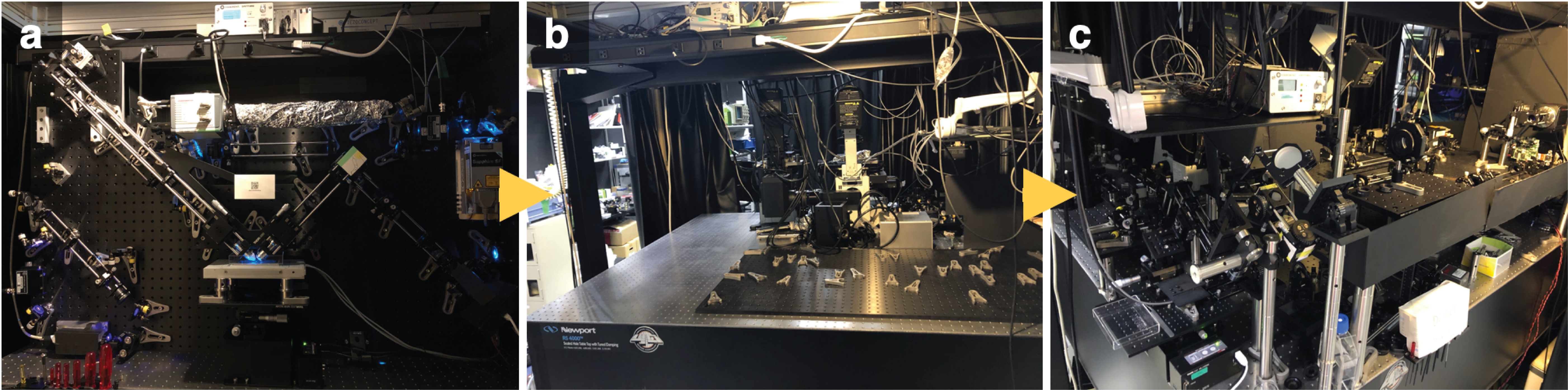
Our initial strategy for this implementation was to place everything on an upright breadboard (Fig. 1a), similar to the configuration used in lattice light sheet microscopy1. However, we encountered a critical vibration issue due to the increased size of the breadboard needed to accommodate both the sheet and structured illumination paths. This technique requires precise overlap of the two illuminations and visualization of fringe patterns, making the elimination of vibration particularly crucial. Despite various countermeasures—such as mounting the detection camera separately to reduce fan vibration, adding anchors with wires to make the breadboard more rigid, and installing a long rod to shift the system’s resonance frequency—vibration persisted, causing relative position changes between the sample and the objective. Consequently, we had to rethink our strategy and dismantle our initial setup (Fig. 1b).
Our modified approach involved the independent installation of the activation objective, excitation/detection objective, sample, and other illumination/detection optics (Fig. 1c). This configuration significantly reduced vibration transmission between components. Only the two objectives and their peripheral components were mounted vertically to illuminate the sample in a water bath, providing more rigid mounting for the other optics. This separation also facilitated space management, allowing us to construct different light sheet modes, such as single- or two-photon activation, and conventional light sheet or scanned Bessel beam. The positional relationship between the sheet illumination, structured illumination, and sample plane was also easier to adjust because each objective was on a three-dimensional stage and movable separately. These modifications resulted in more stable illumination and detection, leading to the first successful SPA-SIM observation.
In observing biological samples, we initially struggled with the mismatch between the focal plane and the plane of the sheet, leading to insufficient background light reduction. Our initial method of adjusting the sheet by observing a fluorescence image of beads embedded in agar gel proved problematic and unsuitable for cell or cell cluster observations. Attempts to use a fluorescent solution also failed. Ultimately, we discovered that the best approach was to adjust the sheet while observing actual cells or cell clusters to achieve optimal optical sectioning. The refraction of the sheet at the surface of cells made the optimal adjustment different from that obtained with gel or solution, necessitating tuning the sheet using the sample being measured. Samples and the optical system are often regarded as separate components, but the optical response from the sample significantly affects the performance of the microscope. This is also the background that allowed the super-resolution microscope to exceed the diffraction limit. Through this research, we were reminded of the need to carefully consider the optical response of the sample in order to take full advantage of the optical system.
We also faced a challenge in implementing two-photon activation SPA-SIM. The RSFPs used in single-photon mode had insufficient activation efficiency, resulting in inadequate signal levels for SIM reconstruction. After extensive screening of our RSFP library, we identified rsGamillus-S, a fluorescent protein with an exceptionally high on-switching rate, which ultimately enabled successful two-photon SPA-SIM imaging. Although rsGamillus-S was not initially developed for this microscopy, our expertise and experience with RSFPs provided the solution.
Finally, we emphasize the critical role of both extensive research experience in a single field and robust interdisciplinary collaborations in developing new methodologies. Several concepts in SPA-SIM were inspired by our prior works. The integration of two-illumination patterns via RSFP, for instance, was based on our previous efforts to enhance resolution in laser-scanning microscopy through nonlinear modification of the point spread function using RSFP2. Our experience in developing the Bessel-beam Raman microscope facilitated the implementation of the Bessel beam for sheet generation3. Additionally, advancements in super-resolution two-photon microscopy directly contributed to reducing sidelobes in two-photon activation4.
Collaboration also significantly enhanced SPA-SIM’s performance. Co-authors from the Nagai group (Kazunori Sugiura, Tomoki Matsuda, and Takeharu Nagai) were instrumental in selecting RSFPs due to their extensive experience in fluorescent protein development. In image reconstruction, Rainer Heintzmann’s algorithm allowed us to adjust parameters based on actual datasets, markedly improving image quality and artifact removal. The successful observation of autophagosomes in MEF cells, in collaboration with Tatsuya Kaminishi, Koki Fukuda, and Maho Hamasaki, further validated the effectiveness of SPA-SIM. These collaborations originated during a young researcher networking event organized by the Institute for Open and Transdisciplinary Research Initiative (OTRI) at Osaka University.
Bridging the gap between conceptual ideas and practical instrumentation is where applied physicists excel. It is akin to discovering a hidden hot spring through persistent effort and transforming it into a soothing spa resort for all to enjoy. Through long-term collaborations with chemists and biologists, we explored numerous aspects of our ideas and transformed them into an exceptional imaging system, SPA-SIM. This system empowers scientists to uncover new phenomena and push the boundaries of their research.
- Chen, B. C. et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998 (2014).
- Kubo, T. et al. Visible-Wavelength Multiphoton Activation Confocal Microscopy. ACS Photonics 8, 2666–2673 (2021).
- Bando, K. et al. Bessel-beam illumination Raman microscopy. Biomed. Opt. Express 13, 3161 (2022).
- Oketani, R. et al. Saturated two-photon excitation fluorescence microscopy with core-ring illumination. Opt. Lett. 42, 571 (2017).
Follow the Topic
-
Nature Methods

This journal is a forum for the publication of novel methods and significant improvements to tried-and-tested basic research techniques in the life sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Methods development in Cryo-ET and in situ structural determination
Publishing Model: Hybrid
Deadline: Jul 28, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in