
Autism spectrum disorder (ASD) is an intractable neuropsychiatric disorder while anxiety disorder is the most common psychiatric disorder in the world. There is increasing evidence that ASD and anxiety are highly comorbid conditions, and understanding the molecular mechanisms of comorbidity is essential for therapeutic strategies for ASD. However, the underlying pathological mechanism of the comorbidity remains unclear. It is of great significance to take into account both molecular signal and circuit regulation for elucidating the pathological mechanism of autism-anxiety comorbidity.
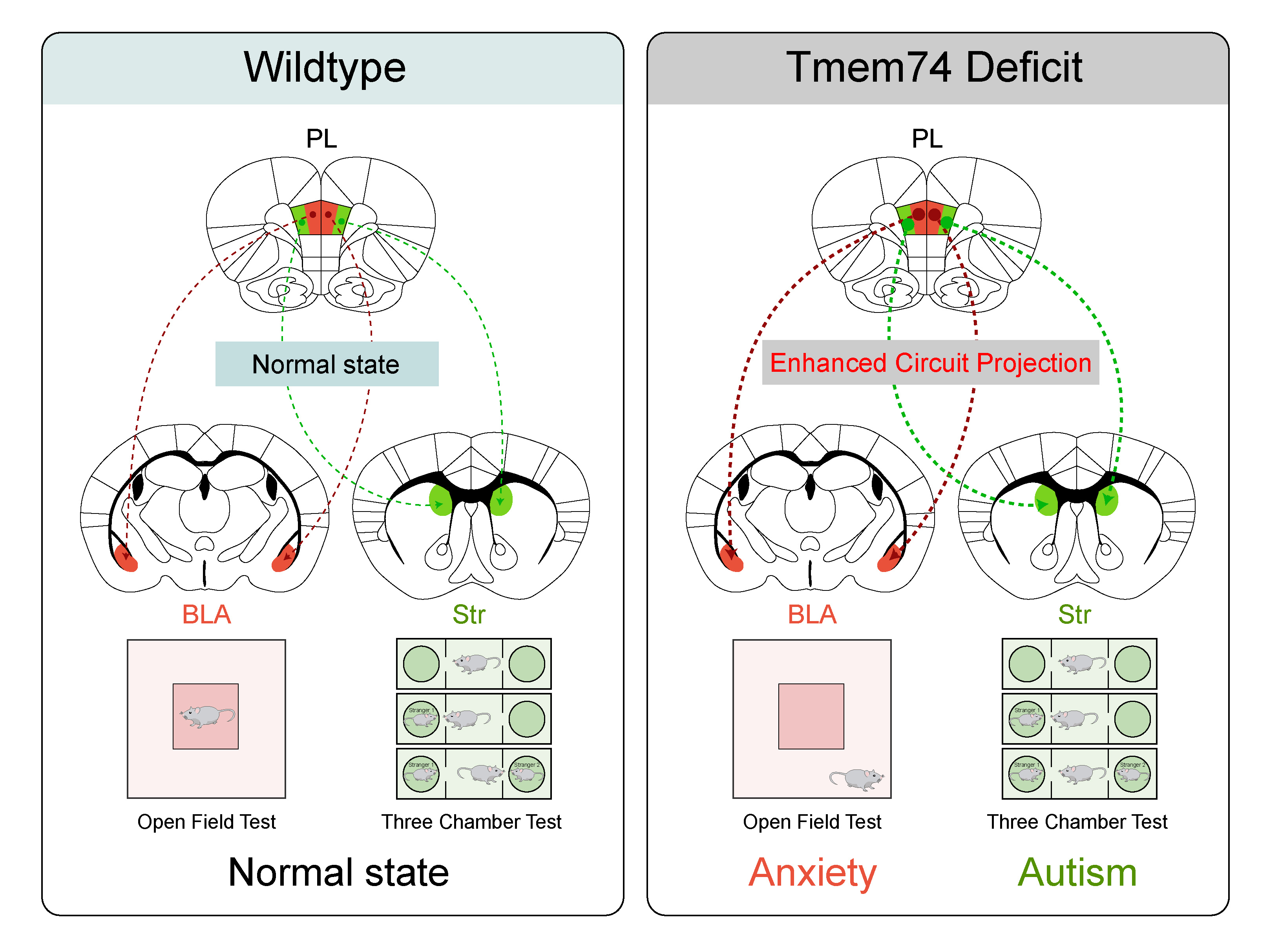
Considering patients with ASD have increased susceptibility to anxiety, it is of great clinical significance to identify hub molecules that could regulate the pathological process of autism-anxiety comorbidity. Utilizing the sequencing results of brain tissue samples from three reported ASD mutant mice, we noticed that the mRNA expression of Tmem74 showed a consistent downward trend in these ASD mutant mice. The remarkable decreased mRNA level of Tmem74 in human pluripotent stem cells (hPSCs) with early autism-related mutation genes further strengthened the strong correlation between Tmem74 and ASD. Together with our previous study that Tmem74 is closely associated with anxiety, we deduced that Tmem74 may serve as a key molecule involved in autism-anxiety comorbidity. To further verify our conjecture, we generated Tmem74-/- mouse model using a TALEN-based strategy. After carrying out a battery of autistic-like and anxiety-like behavioral tests, we confirmed the reliability of Tmem74-/- mice serving as an autism-anxiety comorbidity mouse model. Thus, we provided a brand-new rodent model for further investigation into the pathological mechanisms of autism-anxiety comorbidity.
After performing a series of experiments, we identified that the pyramidal neurons (PNs) in the prelimbic cortex (PL) are critical for regulating the autism-anxiety comorbidity. We further confirmed the increased excitability of PNs in PL of Tmem74-/- mice utilizing whole cell recordings. Reintroduction of TMEM74 into PL via AAV vector reduced the excitability of PNs in PL of Tmem74-/- mice, which could alleviate autistic-like and anxiety-like behaviors. Thus, by combining neurochemistry and neuro-electrophysiology together, we clarified the mechanism that the loss of Tmem74 would enhance the excitability of PNs in PL and then lead to autism-anxiety comorbidity, highlighting the significance of Tmem74 serving as a hub molecule in regulating the comorbidity.
Understanding the connectivity between brain regions and neural circuits is critical for exploring the pathogenesis of autism-anxiety comorbidity. By applying neural tracing technique and optogenetics, we identified two downstream brain regions responsible for autistic-like and anxiety-like behaviors respectively. We discovered that bidirectionally manipulating the excitatory PL-dSTR circuit in CaMKIIα-Cre mice or Tmem74-/- mice could induce or rescue autistic-like behaviors, while the excitatory PL-BLA circuit is responsible for anxiety-like behaviors. Based on these results, we explored the pathogenesis of autism-anxiety comorbidity from the perspective of neural circuits and discovered two brand-new PL-dSTR and PL-BLA circuits mediating autistic-like and anxiety-like behaviors respectively. These findings increase the mechanistic understanding of the PL-related circuits involved in autism-anxiety comorbidity, which might contribute to the development of new therapeutic strategy for autistic patients.
After identifying the two functionally divergent PL projections, we put forward an interesting question that whether one group of PNs in PL participated in the comorbidity coordinatingly or two different groups of PNs in PL mediated distinct neuropsychiatric disorder respectively. In order to make this clear, we adopted two retro-tracing viruses with different colors and injected them into dSTR and BLA respectively (Green into dSTR, red into BLA). After carrying out meticulous analysis, we discovered that the PNs in PL had little overlap between the two colors, and the red was distributed more medially when compared with the green, indicating two anatomically distinct subpopulations of PL pyramidal neurons mediated autistic-like and anxiety-like behaviors respectively. Similar in the electrophysiology properties of the two subpopulations, whether the two subpopulations are molecularly distinct remains to be further explored. Overall, our findings highlighted the importance and the necessity of more precise studies into a brain region for the reason that the neurons within the region could exhibit heterogeneities in terms of morphology, distribution, electrophysiology properties and molecular composition, which might have effects on their functions.
Our study reported a new rodent model of autism-anxiety comorbidity and explored the pathogenesis of autism-anxiety comorbidity from the perspective of molecule and circuit, which might shed light on the treatment of comorbidity via targeting TMEM74 signaling in PL-related circuits. However, several challenges remain in the field. For example, whether the subpopulations in PL are molecularly distinct? What is the specific mechanism regulating the TMEM74 loss-induced hyperexcitability of PNs in PL? What is the protein structural aspects of TMEM74 and its potential significance for the drug design? We are excited to further explore these interesting issues, and multi-disciplinary cooperation offers us considerable promise for answering these questions.
To learn more, please read our scientific article published in Molecular Psychiatry.
Written by
Zhou-yue Wu, Yi-fan Luo, Feng Han, Ying-mei Lu
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in