The morphological roadmap of development: How do cellular shapes change over embryogenesis?
Published in Research Data and Cell & Molecular Biology

Featured Image: A beguiling smile 'face' of a roundworm Caenorhabditis elegans embryo, depicted at the center in a dorsal view with the anterior oriented downward. The image showcases primarily skin cells (red) within a ~400-cell-stage embryo. A mosaic of other cell types is interwoven and colored in gray, green, and blue, or presented in a translucent manner for visual emphasis. Encircling this central image are confocal fluorescence snapshots showcasing GFP-highlighted cell nuclei and mCherry-highlighted cell membranes (internal) and reconstructed cellular morphologies (external). These concentric frames capture the transformative journey of the C. elegans embryo, progressing from two cells to over 550 cells. Bridging the developmental timeline is a series of merged GFP and mCherry fluorescence images that narrate the transition to the intricate anatomy of a mature C. elegans adult.
A morphological roadmap of worm embryogenesis
Morphology or shape is the fundamental geometric and physical property of cells, which are commonly coupled with their normal functions. For example, the irregular shapes of invasive cancer cells are indicative of their potential in metastasis, while the elongating shape of nerve cells are indicative of their potential in establishing a connection during development. Learning morphological dynamics of cells, tissues, and organs is invaluable for both fundamental research and clinical diagnostics. By analyzing the time-lapse images of these biological samples during development or disease progression, researchers could detect abnormalities in the formation of cell/tissue types or disease progression in the first place, which helps develop new therapeutic interventions.
Recently, through the years of seamless collaboration between the laboratories of Drs. Chao Tang (汤超) at Peking University, Zhongying Zhao (赵中应) at Hong Kong Baptist University, and Hong Yan (严洪) at City University of Hong Kong, they have established a new platform that allows quantitative delineation of the dynamics of cellular shapes throughout C. elegans embryogenesis, which was published online in Nature Communications, titled “Cell lineage-resolved embryonic morphological map reveals signaling associated with cell fate and size asymmetry” [1]. In this article, Guan et al. utilizes a small worm, C. elegans, to investigate how the cellular shapes change over tissue formation and organogenesis during embryonic development with resolved cell lineage (Fig. 1; Fig. 2). The customized algorithm and the resolved cellular shape dynamics lay a foundation not only to study the cellular morphological changes in a wild-type developing embryo, but also permit the delineation of cell shape dynamics under genetic perturbations, mechanical compression, cell ablation, or stress conditions.
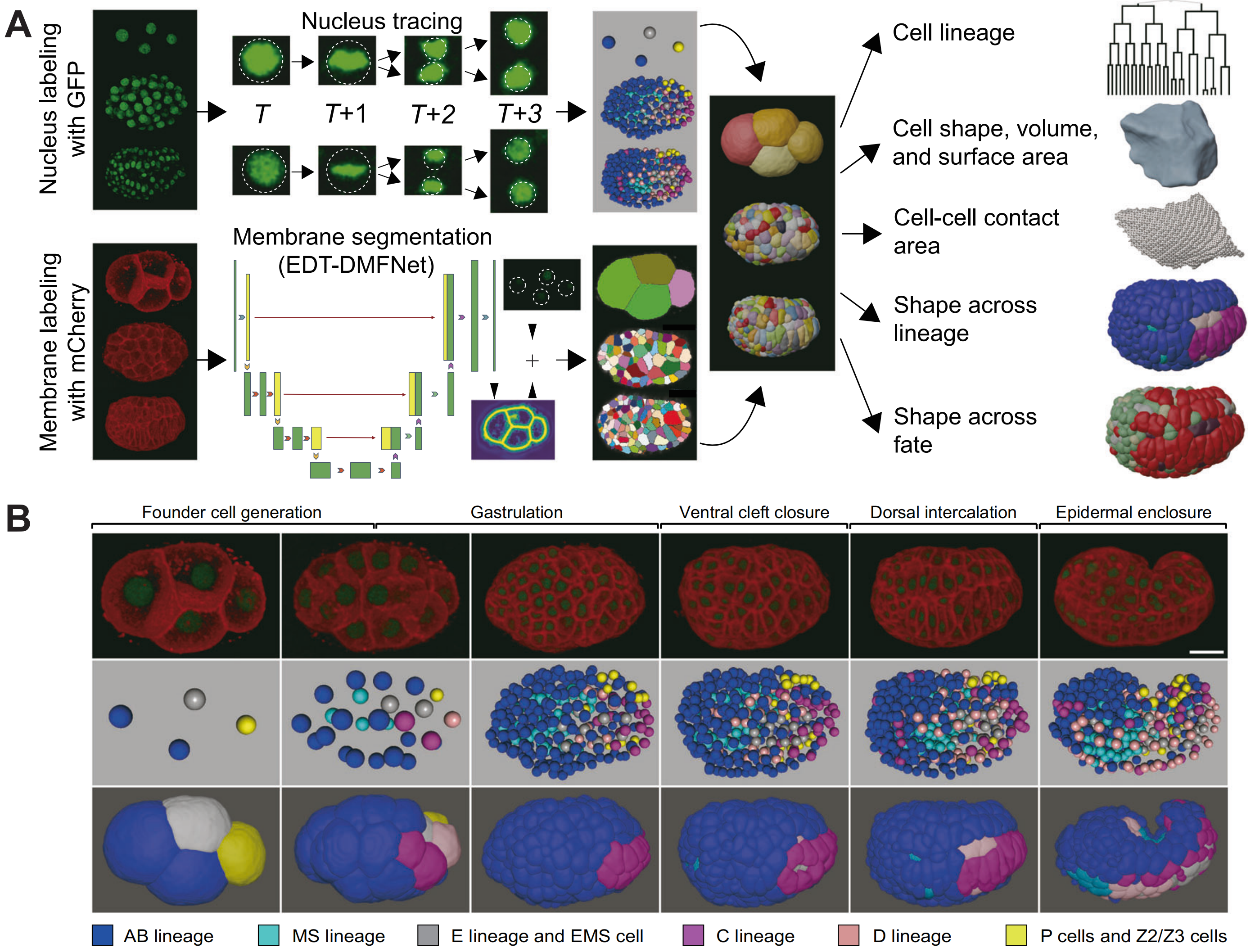
Fig. 1. (A) An in vivo and in silico framework for quantifying a worm embryo’s cell-resolved morphologies. (B) A worm embryo’s cell-resolved morphologies in different lineages and at different stages.
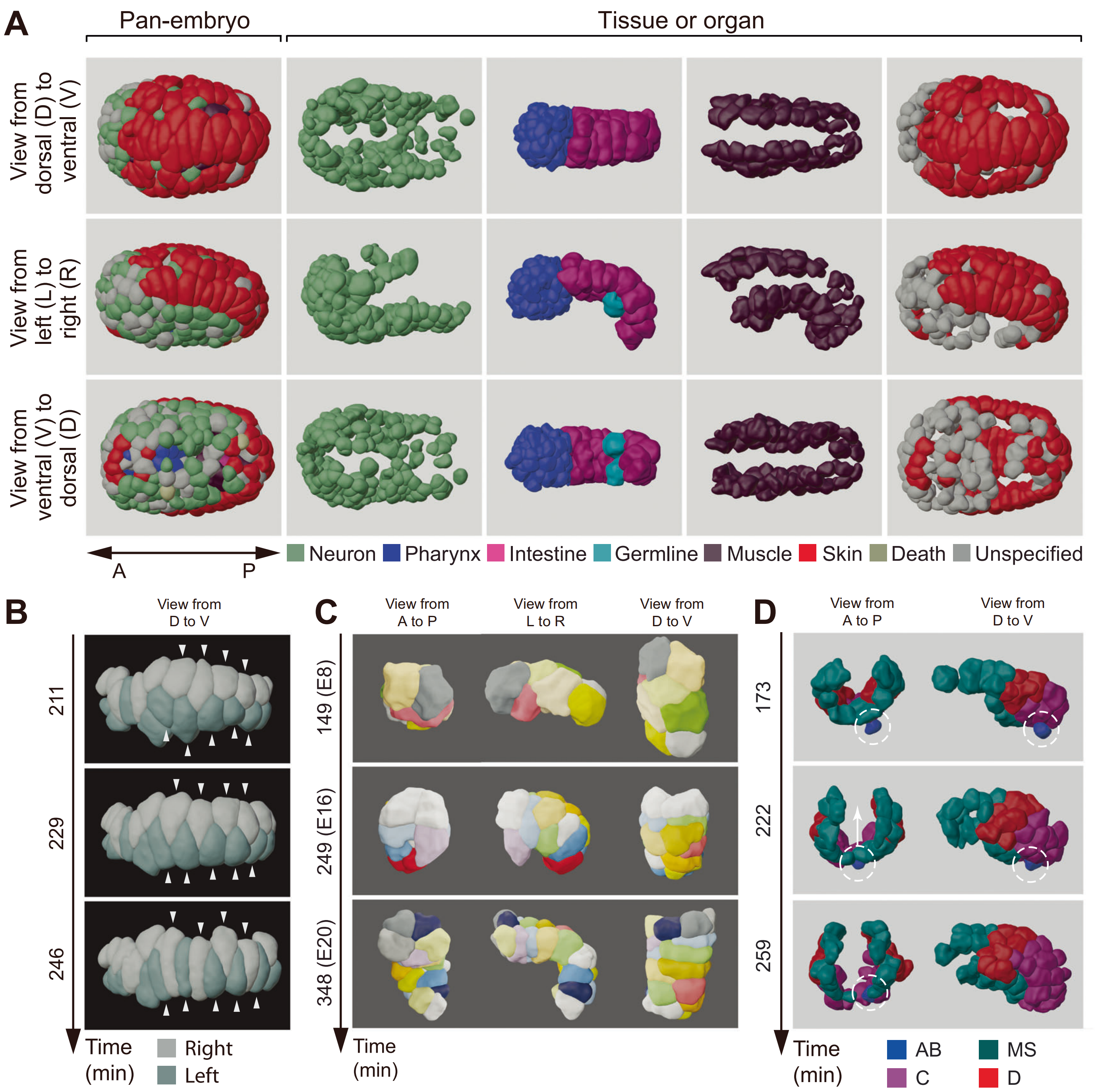
Fig. 2. (A) A worm embryo’s cell-resolved morphologies in different tissues and organs. (B) Morphological change of dorsal skin over time. (C) Morphological change of intestine over time. (D) Morphological change of body-wall muscle over time.
A multidisciplinary team consisting of biologists, physicists, and computer scientists
Five years ago, the research team published the first morphological roadmap from worm fertilized egg up to its mid-development [2]. In this study, they picked up where that work left off, charting the second half of the worm’s journey as cells differentiate, migrate, deform, and organize into a pre-hatching larva.
What makes this advance possible is the seamless collaboration between three laboratories with very different expertise:
- Zhongying Zhao is a geneticist with expertise in worm genetics and genomics, transgenesis and gene editing, as well as automated cell lineage tracing during embryogenesis.
- Hong Yan is a computer scientist whose expertise in computer vision, image processing, and machine learning enables the development of various algorithms with wide use in both academic and industry applications.
- Chao Tang is a physicist whose pioneering research into complex systems — represented by the Bak-Tang-Wiesenfeld model developed in the 1980s — has shown how simple, local interaction rules can give rise to rich patterns and self-organized criticality [3].
Fifteen years ago, when Prof. Zhao set up his lab, he began amassing time-lapse 3D images of thousands of developing worms. Computer scientists and physicists were intrigued by the high-quality data, but early collaborations remained stovepiped — each discipline tackled the data in its own way. That started changing in 2012 when he teamed up with computer scientist, Prof. Yan. Their progress on cell lineage data analysis has accelerated since the involvement of Prof. Tang’s lab. He recruited Dr. Guoye Guan, who was then an undergraduate in physics, had a strong interest in biology and got admitted to the Center for Quantitative Biology at Peking University. Guoye was captivated by Prof. Tang’s groundbreaking research on self-organized criticality and biological design principles and was eager to pursue his Ph.D. in Tang’s lab. To prepare him, Tang invited Guan to apply a quantitative, theory-driven lens to the rich datasets assembled by Prof. Zhao’s lab.
During Guan’s first visit to Hong Kong, he met the other enthusiastic youngsters, Jianfeng Cao (a Ph.D. student majored in computer science from Yan’s lab), Wincy Wing Sze Ho, MingKin Wong, and LuYan Chan (Ph.D. and M.Sc students majored in genetics and developmental biology from Zhao’s lab), who later became the authors of the three labs’ first joint paper about the morphological roadmap in the first half embryonic development [2]. As the old saying goes, two heads are better than one. They quickly learned that the hardest biological puzzles often yield only when examined through multiple lenses. “Aha~ it turns out we needed all biologists’ experiments, computer scientists’ algorithms, and physicists’ analyses together,” recalls MingKin (Fig. 3). Addressing complex biological questions requires experts from diverse fields to work hand in hand — collecting, processing, and interpreting data through both experimental and computational approaches. Progress in any one step fuels improvements in the others, creating a virtuous cycle that continually strengthens the entire data ecosystem.

Fig. 3. Prof. Zhongying Zhao (Left), Dr. Guoye Guan (Middle), and Dr. MingKin Wong (Right) presenting their project in the 19th International C. elegans Conference, photographed in the summer of 2019.
Data collection, maintenance, and sharing to researchers across all fields
On top of the morphological roadmap, the research team further integrated cell-resolved expression profiles for >400 genes from previous literature and new experiments. All data can be accessed via the ITK-SNAP-CVE software (C. elegans virtual embryogenesis; https://doi.org/10.6084/m9.figshare.24768921) and CMOS website (cellular morphology of C. elegans embryo; https://bcc.ee.cityu.edu.hk/cmos) (Fig. 4; Fig. 5). Now every two years, Prof. Zhao leads the three labs’ seniors to give hands-on teaching in EMBO Practical Course | C. elegans: from genome editing to imaging, held by the European Molecular Biology Laboratory (EMBL) (https://www.embl.org/about/info/course-and-conference-office/events/cel24-01). This in-person and online course helps participants all over the world to use their up-to-date tools and data for studying their biological questions of interest.
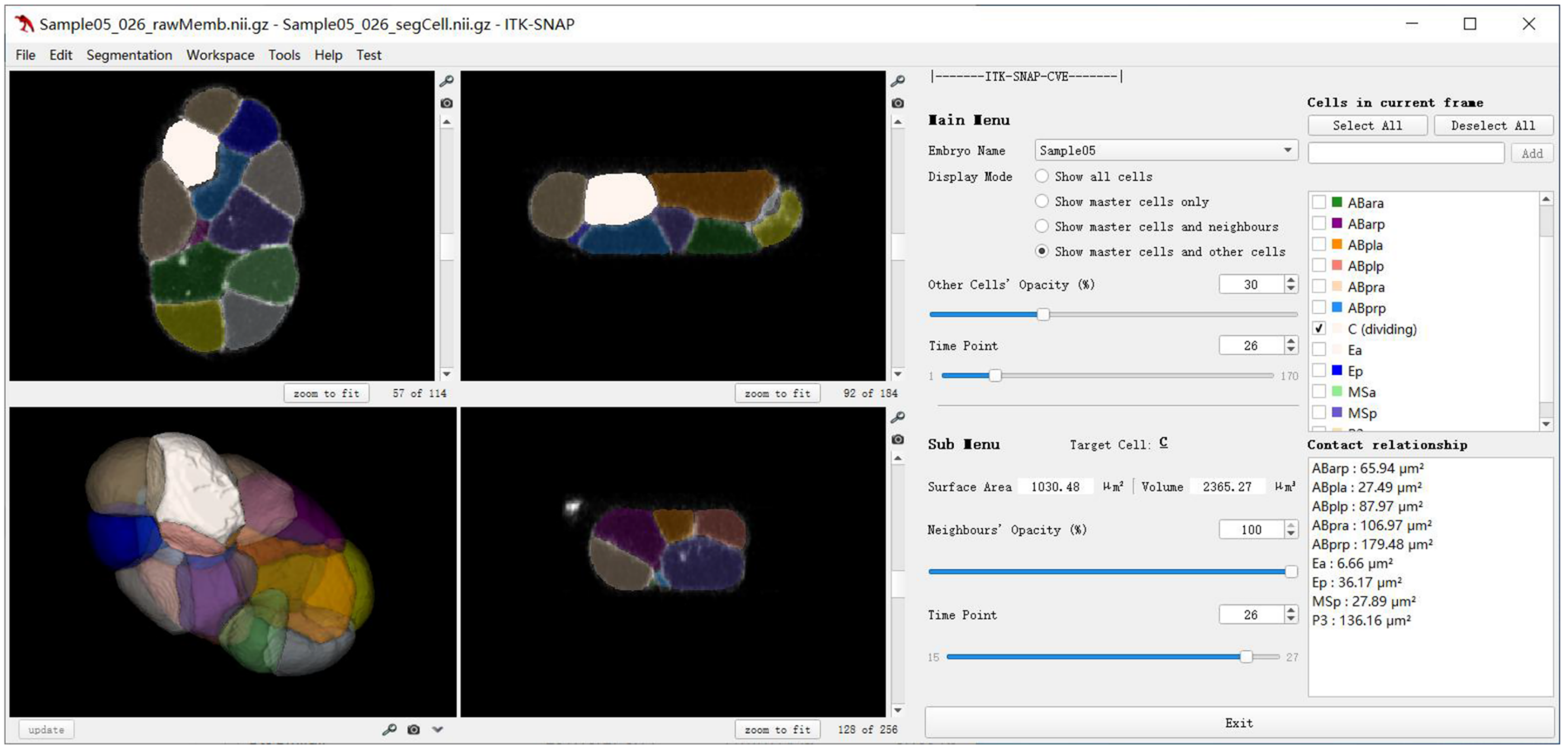
Fig. 4. The user interface of ITK-SNAP-CVE software.
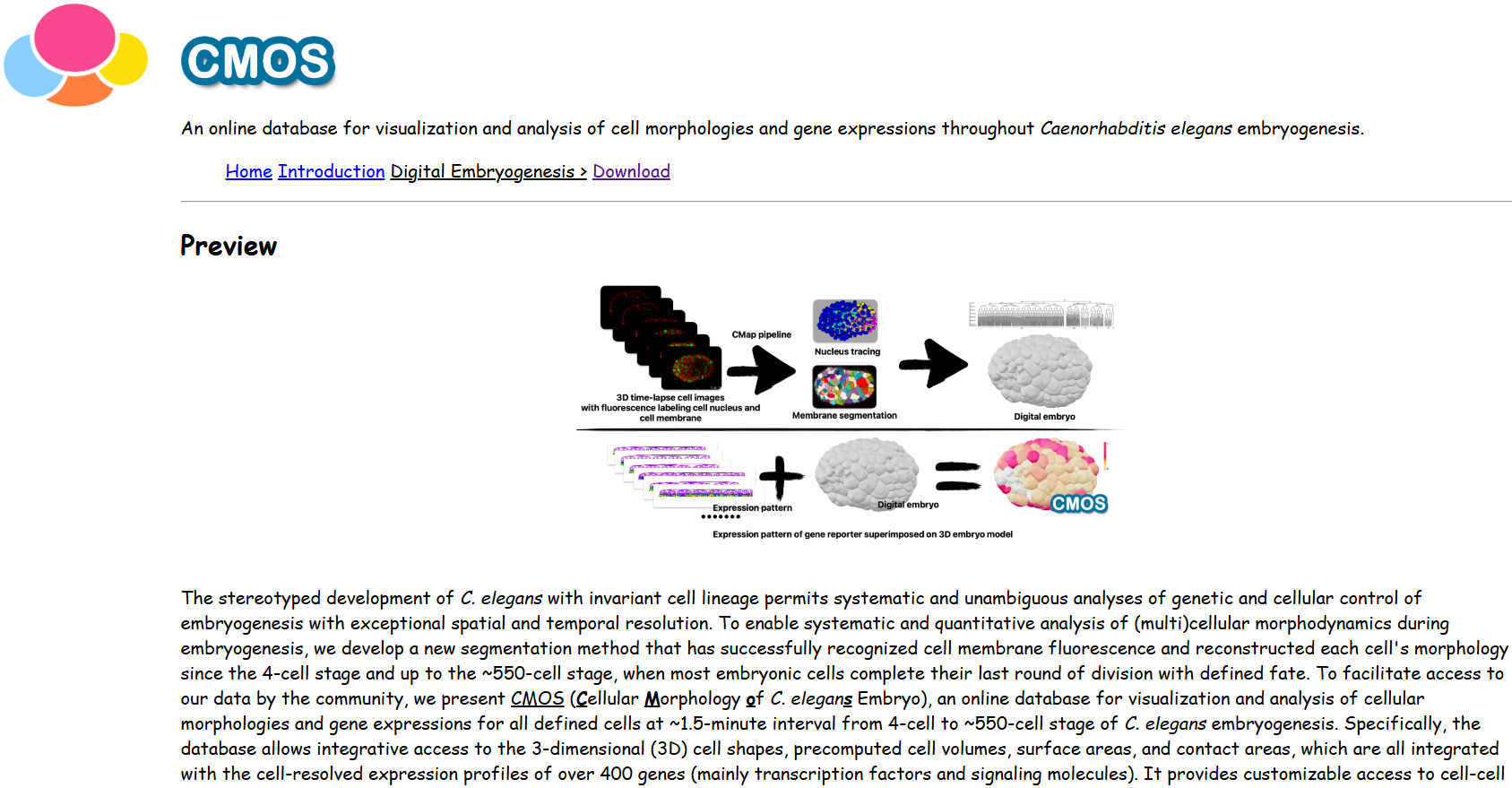
Fig. 5. The user interface of CMOS website.
Today, the three labs have not only developed a long‑term partnership in data mining and host regular exchange visits (Fig. 6), but also spearhead advances across their respective fields: long-duration 3D live cell imaging [4-7], computer vision [8-11], physics of life and biological design principles [12-15], and advanced developmental data analysis [16-19]. Looking ahead, the team envisions these high‑quality biological datasets enabling cross-disciplinary studies from developmental biology, biomechanics to AI-based image analysis.
Fig. 6. The three professors and their Ph.D. students having breakfast in Hong Kong, photographed in the summer of 2023.
References
[1] https://doi.org/10.1038/s41467-025-58878-0
[2] https://doi.org/10.1038/s41467-020-19863-x
[3] https://en.wikipedia.org/wiki/Abelian_sandpile_model
[4] https://doi.org/10.1534/genetics.118.300820
[5] https://doi.org/10.1186/s12859-019-2720-x
[6] https://doi.org/10.3389/fcell.2022.978962
[7] https://doi.org/10.1093/bioinformatics/btae626
[8] https://doi.org/10.1109/SMC54092.2024.10831421
[9] https://doi.org/10.1109/ICIP51287.2024.10648269
[10] https://doi.org/10.1109/TCBBIO.2025.3542123
[11] https://doi.org/10.48550/arXiv.2503.02261
[12] https://doi.org/10.1103/PhysRevE.104.054409
[13] https://doi.org/10.1371/journal.pcbi.1009755
[14] https://doi.org/10.1016/j.cnsns.2022.106966
[15] https://doi.org/10.1038/s41540-023-00265-w
[16] https://doi.org/10.1039/c5mb00417a
[17] https://doi.org/10.1093/bioinformatics/btw796
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in