The power of aqueous chemistry for unveiling metastable solids and nanomaterials: h'-WO3
Published in Chemistry

This discovery originates from an exploratory work funded by SOLVAY company and whose aim was to investigate metal oxide nanomaterials, bearing transition metal cations with mixed valence in order to provide innovative solutions to the energy conversion field. Few years before, between 2011 and 2014, we had acquired expertise in the design of nanostructured mixed valence titanium oxides, such as Magnéli phases TinO2n-1, (n ≥ 4), where the combination of the nanoscale with mixed valence and specific crystal structures had yielded interesting thermoelectric (Portehault et al.) and resistive switching (Maneeratana et al.) properties. For the present work, we have studied tungsten-based oxides with mixed valence W6+/W5+ because they represent a wide range of compounds with interesting properties. Our ambition was to explore domains of synthesis conditions that were untouched yet in order to unveil, possibly, new compounds and new nanomaterials.
We decided to focus on aqueous chemistry in soft conditions, so-called “Chimie douce”, i.e. temperature below 100 °C and water as solvent. Soft chemistry, one of the foundations of our academic team Nanomaterials in the Lab. Chimie de la Matière Condensée de Paris, provides a range of experimental levers in order to tune crystallization pathways, such as pH, redox potential, temperature, time and surface ligands, while approaching green chemistry conditions. These soft synthesis conditions are especially well suited to isolate metastable solids and nanostructures that exhibit a large contribution of the surface to the total energy. In order to identify conditions of synthesis that could be fruitful for the discovery of new materials, we examined the Pourbaix diagram of tungsten in water and detected a narrow range of pH and redox conditions where mixed valence oxides existed. Noteworthy, even if this diagram shows thermodynamic phases, it provided a good starting point to target kinetic products. This is how we set the stage for the discovery of new tungsten-based oxide nanomaterials.
By using this soft chemistry strategy, we readily isolated a new solid in the form of nanoplatelets. According to the transmission electron microscopy images, an initial crystal structure model could be easily proposed and then refined based on X-ray diffraction, X-ray photoelectron spectroscopy and elemental analyses. We then identified this new material as a proton bronze of a new form of tungsten trioxide, h’-H0.07WO3, with royal blue color typical of the mixed valence W6+/W5+.
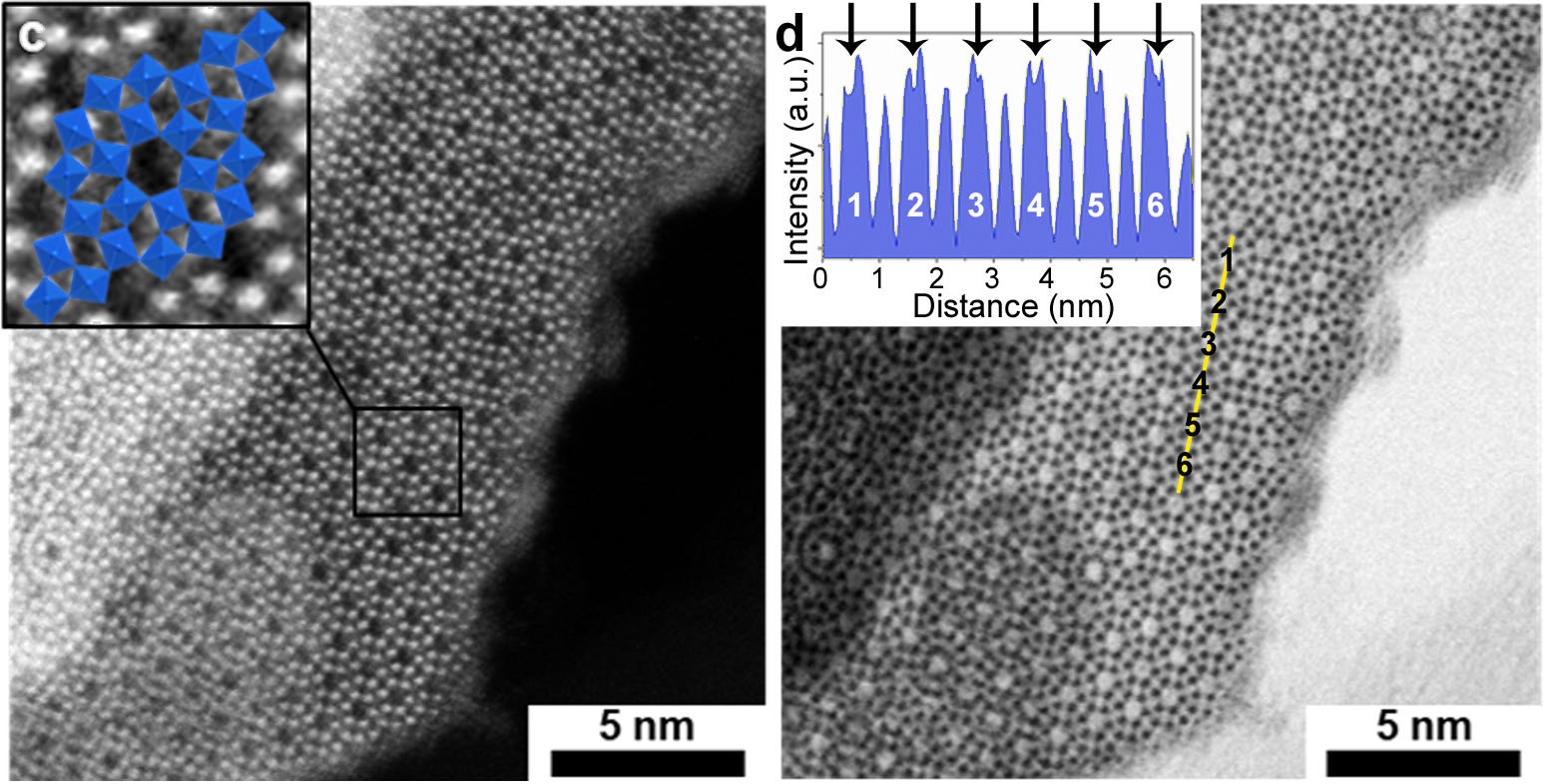
This finding of a new compound was soon followed by a second surprise: the ability of the nanomaterial to release its proton under gentle heating, yielding stoichiometric white h’-WO3. The discovery of new compounds with such simple compositions and at near-ambient conditions is a rare event nowadays and it made our days for a significant time. The third astonishing observation came when the material was implemented in simple electrochromic devices and showed very convincing performances.
After these discoveries, the material and its synthesis protocol were patented. They have now been published in Nature Communications. The material is now for us a perfect platform to explore further its mixed valence-related properties, but also to target new materials by adapting the synthetic conditions.
This research is now accessible at:
https://www.nature.com/articles/s41467-018-07774-x
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in