The REZPEG Story
Published in General & Internal Medicine, Immunology, and Anatomy & Physiology
Psoriasis (PsO) and atopic dermatitis (AD) are among the most common T cell-driven inflammatory skin disorders that can significantly impact quality of life1. While recently developed immunosuppressive biologic therapies have been successful in targeting the specific immune pathways dysregulated in each disease, unpredictable or incomplete responses, side effects, and relapse remain as challenges2.
Regulatory T cells (Tregs) are pivotal in orchestrating immune homeostasis through T helper cell modulation, but are impaired in many autoimmune and chronic inflammatory diseases including PsO and AD3,4. Recognizing that interleukin-2 (IL-2) plays a role in controlling the proliferation and survival of Tregs5, we explored the ability of an IL-2-based therapy to restore Treg immunological balance in these cutaneous disorders. This idea was supported by previous studies showing that low-dose IL-2 was able to partially rescue Treg function and provide clinical benefit in autoimmune diseases6. Low-dose IL-2, however, has a narrow therapeutic window and short half-life, requiring more frequent dosing that could limit clinical practicality as well as result in undesirable CD4 and CD8 T cell induction.
In designing an IL-2-based biologic, we started with the approved recombinant human IL-2 (rhIL-2) aldesleukin protein sequence and generated structurally different drug candidates by conjugation with stable, covalently attached polyethylene glycol (PEG) moieties7. We used in vivo screening to simultaneously evaluate the on-target effects (magnitude and duration of elevation of Tregs), off-target toxicity (levels of conventional CD4 and CD8 T cells and other lymphocyte/granulocyte populations), and the pharmacokinetic profile8.
This resulted in the selection of rezpegaldesleukin (REZPEG) as the lead drug candidate. REZPEG has an extended half-life as well as a selectivity for Treg stimulation over conventional T cells compared with rhIL-28. The benefits of its unique design were intended to enable the effective management of diseases involving Treg dysregulation with infrequent dosing regimens to improve clinical tolerability and patient convenience and compliance7.
In addition to inducing robust Treg expansion in mice and monkeys, REZPEG also maintained Treg elevation in a mouse model of systemic lupus erythematosus (SLE), an autoimmune disease associated with reduced Tregs, and ameliorated disease progression8. Data from proof-of-concept clinical trials confirmed Treg expansion and an extended pharmacokinetic/pharmacodynamic profile. In this short, 6-week Phase 1b trial in patients with mild-to-moderate SLE, REZPEG showed rapid and dose-dependent reductions in Cutaneous Lupus Erythematosus Disease Area and Severity Index-Activity (CLASI-A) scores5. Additional nonclinical studies using a classic preclinical model of cell-mediated immunity confirmed that REZPEG reduced cutaneous inflammation and induced antigen-specific immune tolerance in mouse skin. These results encouraged us to initiate clinical trials in patients with inflammatory cutaneous diseases, leading to our recent publication in Nature Communications9.
Here, in two randomized, double-blind, placebo-controlled Phase 1b trials in patients with AD or PsO (NCT04081350 and NCT04119557), REZPEG was administered subcutaneously every 2 weeks through week 12, with post-treatment follow-up visits continuing through week 19, and extended visits for week 19 responders continuing through week 48. In both studies, REZPEG was safe and well-tolerated, demonstrating consistent pharmacokinetics and clinical efficacy.
At the 12-week key efficacy endpoint, we were excited to see that PsO patients treated with 24 µg/kg REZPEG demonstrated improvements from baseline in Psoriasis Area and Severity Index. Additionally, AD patients

Figure 1 | Atopic dermatitis investigator-assessed and patient-reported efficacy outcomes. Mean percent change from baseline in a Eczema Area and Severity Index score, b body surface area score, and c Dermatology Life Quality Index.
Consistent with REZPEG’s biological activity as an IL-2 receptor agonist, these clinical improvements were accompanied by sustained increases in CD25bright Tregs with no significant changes in CD4 or CD8 conventional T cells over the 12-week treatment period. Interestingly, CD56+ natural killer (NK) cells were also increased over the treatment period, consisting of an increase in CD56bright and a decrease in CD56dim NK cell subsets, which could contribute to REZPEG’s immunotherapeutic effect.
To investigate REZPEG’s biomolecular mechanisms in AD beyond Treg pharmacodynamics, a proteomic analysis was conducted. We observed modulation of immunoregulatory processes (interleukin, TNF superfamily, and chemokine signaling), cellular migration/adhesion networks (integrins, extracellular matrix organization, and cell surface interactions). We were encouraged to see that REZPEG also reduced the expression of serum proteins known to be elevated in AD patients and also demonstrated an effect on the expression levels of known targets for current AD therapy.
Taken together, we propose a potential mechanism of action for REZPEG involving the engagement of multiple immunoregulatory mechanisms that facilitate the attenuation of Th1, Th2, and Th17 responses through Treg expansion (Figure 2). This is exciting, since REZPEG appears to be uniquely poised to address a broad range of immunopathologies through a central pathway of IL-2 receptor-driven Treg rescue, which contrasts with many of the currently available pharmacological agents that have a disease-limited, narrow biological target.
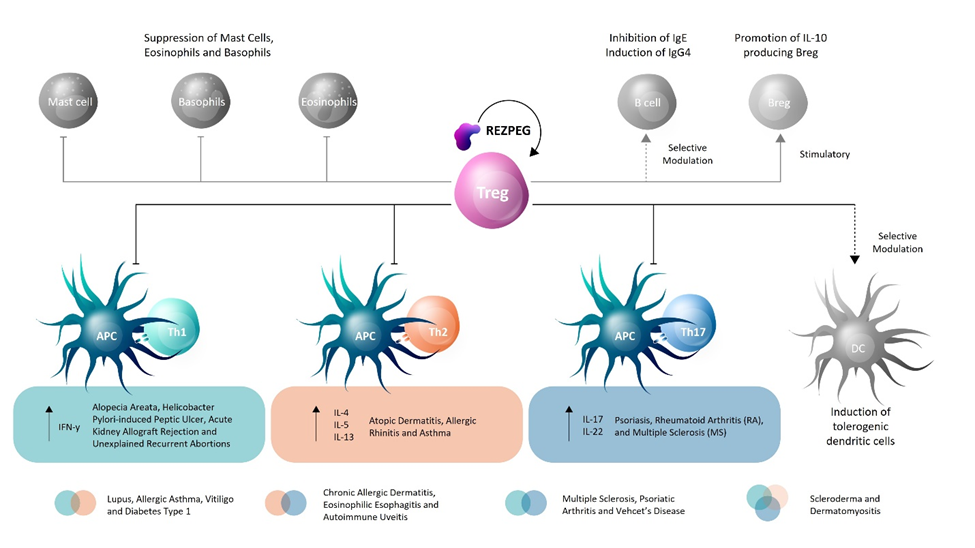
Figure 2 | REZPEG mechanism of action hypothesis in restoring Treg homeostasis in inflammatory disorders.
These results help validate a role for Tregs in chronic inflammatory skin conditions and support continued evaluation of REZPEG’s clinical potential in cutaneous disease treatment.
REFERENCES
1. Chen, W. Y. et al. Annoying Psoriasis and Atopic Dermatitis: A Narrative Review. Int. J. Mol. Sci. 23, 4898 (2022).
2. Ujiie, H. et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 9, 875492 (2022).
3. Sakaguchi, S. et al. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 38, 541-566 (2020).
4. Nedoszytko, B. et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part II: The Treg role in skin diseases pathogenesis. Postepy. Dermatol. Alergol. 34, 405-417 (2017).
5. Fanton, C. et al. Selective expansion of regulatory T cells by NKTR-358 in healthy volunteers and patients with systemic lupus erythematosus. J. Transl. Autoimmun. 5, 100152 (2022).
6. Abbas, A.K. et al. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 3, eaat1482 (2018).
7. Fanton, C. & Zalevsky, J. Recent patents in allergy and immunology: The interleukin-2 receptor pathway agonist rezpegaldesleukin (REZPEG) for the rescue of regulatory T cells in chronic inflammatory and autoimmune diseases. Allergy.79, 2565-2566 (2024).
8. Dixit, N. et al. NKTR-358: A novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J. Transl. Autoimmun. 4, 100103 (2021).
9. Silverberg, J.I. et al. The regulatory T cell-selective interleukin-2 receptor agonist rezpegaldeseukin in the treatment of inflammatory skin diseases: two randomized, double-blind, placebo-controlled phase 1b trials. Nature Communications. 15, 9230 (2024).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in