The Role of the Windsor Framework in Northern Ireland: Redefining Medicine Supply in a Divided Market
Published in Social Sciences, Pharmacy & Pharmacology, and Economics
Our journey began with a curiosity about how medicines reach patients, particularly in areas with complex political and regulatory environments. Northern Ireland(NI) is one such region that faced major challenges in the supply of medicine following Brexit. Though NI was part of the United Kingdom (UK), it shared a border with the Republic of Ireland, a European Union member state. Because of this situation, NI had to navigate a dual regulatory system. This meant that a single medicinal product had to comply with both MHRA (UK) and EMA (EU) requirements, which created confusion after Brexit, especially in how medicines were approved, labelled, and supplied to NI.
The Windsor Framework was announced in February 2023 to address these challenges and came into effect from 01 January 2025. This new agreement between the UK and EU aims to provide regulatory clarity and smoother access to medicines throughout the UK, including Northern Ireland.
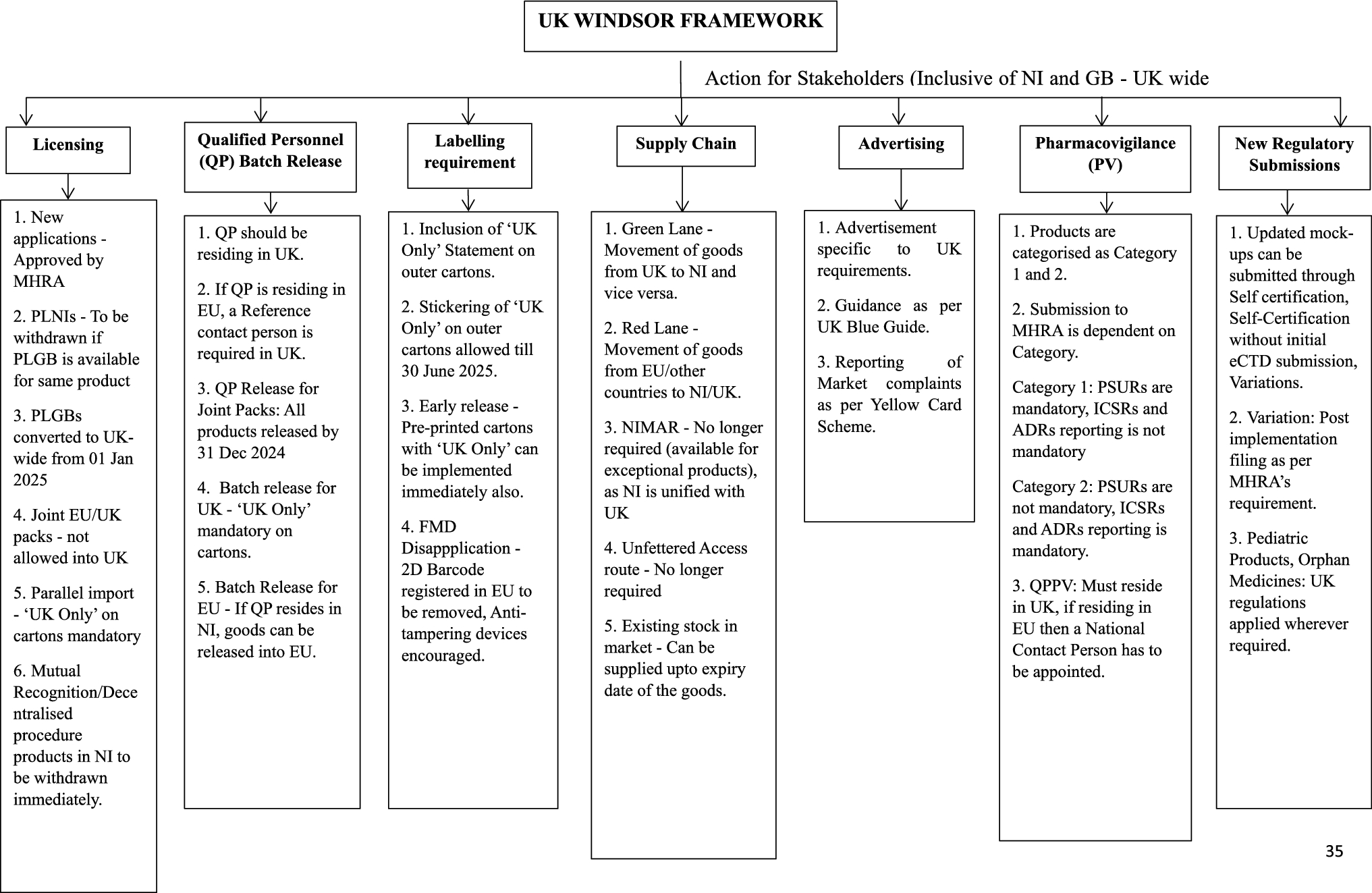
Our recently published paper in Therapeutic Innovation & Regulatory Science (https://doi.org/10.1007/s43441-025-00753-7) provides a comparative analysis of how medicine regulations in NI have changed before and after the implementation of the Windsor Framework, focusing on areas such as licensing, QP batch release, labelling, supply routes, advertising and pharmacovigilance reporting. This review is a practical guide for stakeholders such as manufacturers, Marketing Authorisation Holders and regulatory professionals to understand the regulatory changes and their practical implications.
Need for the Windsor Framework:
- To avoid a hard border between NI and the Republic of Ireland while maintaining the integrity of the EU single market.
- To resolve dual regulatory conflicts where medicines in NI had to meet the MHRA (UK) and EMA (EU) regulations, leading to delayed approvals of new medicines and duplication of existing data.
- To address medicine shortages caused by disrupted supply chains and complex import requirements post-Brexit.
- To simplify licensing and labelling processes and reduce the administrative burden on pharmaceutical manufacturers and wholesalers operating across the UK and EU markets.
- To restore patient access and public confidence by ensuring a faster and more reliable supply of medicines throughout the UK, including NI (1)
Impact of the Windsor Framework:
The key improvements of the Windsor Framework include:
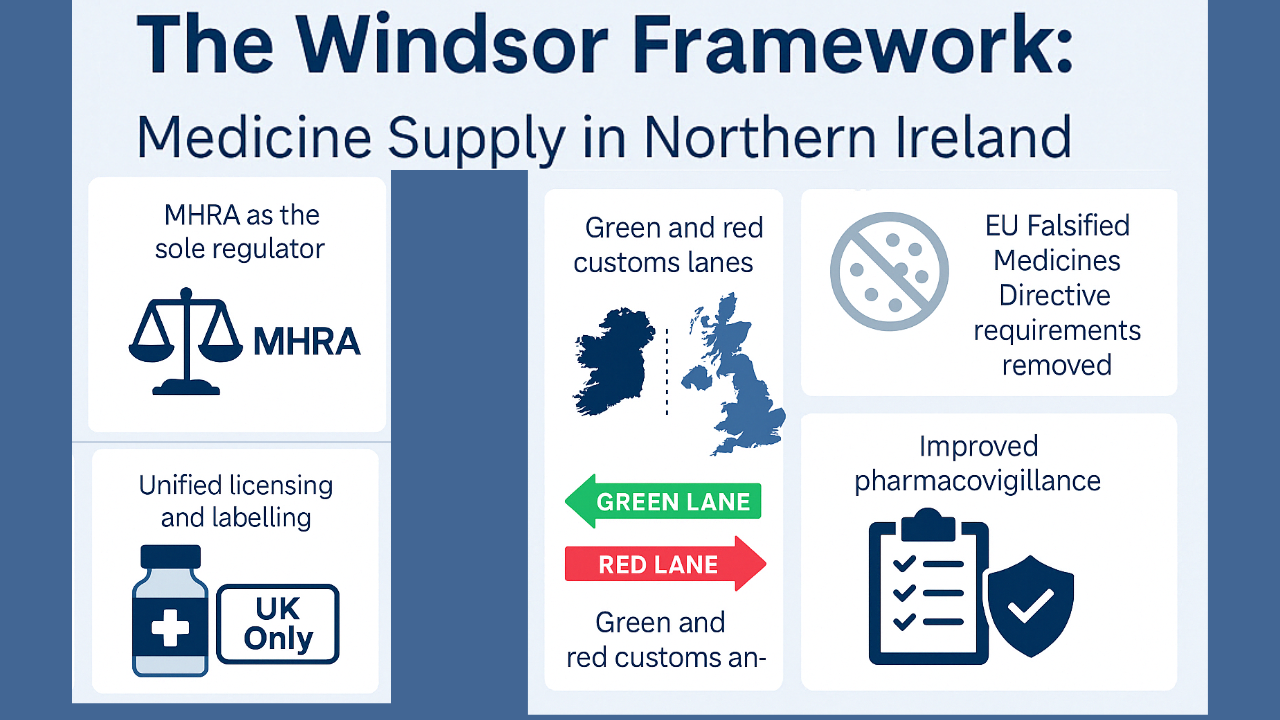
- MHRA becomes the sole authority: MHRA is now the sole regulatory authority for approving medicines across the UK (including NI). There is no longer a need to comply with overlapping EU regulations.
- Unified Licensing and Labelling: A medicinal product no longer requires two marketing authorisations (one from the MHRA and one from the EMA). Instead, a UK-wide license can be mandatorily used with a ‘UK Only’ statement on outer packs.
- Falsified Medicines Directive (FMD) features are no longer mandatory: The EU FMD, which required barcodes and other serialisation features, has been disapplied in NI and has to meet UK safety requirements.
- Simplified Customs: Green & Red Lanes: A green lane has been introduced for products moving within the UK, i.e., between Great Britain and Northern Ireland or vice versa, which are subjected to fewer checks. The red lane system is for products moving between the EU and UK (including NI) subject to stricter checks.
- Pharmacovigilance: Pharmacovigilance and safety reporting are now fully monitored by the UK’s MHRA system(2).
The Future of the Windsor Framework:
The Windsor Framework was not only introduced to streamline the process of movement of goods across NI–GB–EU, but it was also intended to reduce the political stress induced due to Brexit. The shared island of Ireland has been in a chaotic environment since 2020. However, the NI/Ireland protocol was introduced to avoid a hard border between the two countries; the failure to completely implement the protocol increased both countries' political and cultural tensions. Hence, the Windsor Framework is expected to foster a more united and peaceful relationship among the UK, NI and the EU. Looking ahead, the framework may shape NI’s role in different ways. Some foresee the possibility of a united Ireland, while others warn of the NI area becoming politically isolated if the internal tensions in the UK grow further. Alternatively, NI may continue to hold a special status, benefiting from links to the UK and EU. Though challenges remain, the framework offers a chance to build stability and a foundation for cooperation in a complex post-Brexit landscape(3).
References:
- Northern Ireland Protocol: The Windsor Framework - House of Commons Library [Internet]. [cited 2025 Apr 14]. Available from: https://commonslibrary.parliament.uk/research-briefings/cbp-9736/
- The Windsor Framework - further detail and publications - GOV.UK [Internet]. [cited 2025 Apr 14]. Available from: https://www.gov.uk/government/collections/the-windsor-framework-further-detail-and-publications
- Diamond P, Colfer B. THE WINDSOR FRAMEWORK AND ITS IMPLICATIONS. 2024 [cited 2025 Apr 15]; Available from: www.triptyque.be
Follow the Topic
-
Therapeutic Innovation & Regulatory Science

This is a peer-reviewed journal that focuses on medical product discovery, development, regulation, access, and policy.
Related Collections
With Collections, you can get published faster and increase your visibility.
Patient Preferences to Inform Decision Making in Medical Product Development
Therapeutic Innovation and Regulatory Science (TIRS) is calling for papers for a special collection entitled Patient Preferences to Inform Decision Making in Medical Product Development.
The number of patient preference studies related to treatment choice has increased substantially in recent years. Many of these studies focus on eliciting patients’ preferences for attributes of treatments that have recently received or soon will be considered for marketing authorization by regulators. Applications of preference methods to the myriad decisions that sponsors encounter earlier in clinical development have been fewer in number.
This special collection will focus on applications of patient preference methods to elicit patient input to inform clinical development decisions. Such decisions include, but are not limited to: endpoint identification and selection, clinical trial design, clinically meaningful changes in outcomes, target product profiles, and weighting for composite endpoints and preference-weighted PROs.
Papers will be considered if they address explicitly decisions that sponsors, regulators, and other decision makers need to make prior to an application for market authorization. Although theoretical and methods papers will be considered, preference will be given to empirical applications.
For questions regarding suitable content, submission status, and guidelines, please email Dr. Sandra Blumenrath, Managing Editor of TIRS, at Sandra.Blumenrath@diaglobal.org.
Publishing Model: Hybrid
Deadline: Ongoing
The Inflation Reduction Act and Its Impact on Innovation, Access, and Affordability
Signed into US federal law in 2022, the Inflation Reduction Act (IRA) represents an unprecedented shift in healthcare policy, particularly concerning the Government’s role in setting drug prices for the Medicare program and the consequential impact on pharmaceutical innovation, access, and patient affordability. Rigorous research is needed to inform policymakers, industry leaders, and the public about the IRA’s potential impact on the development of and access to critical medical advances and devise strategies to mitigate any adverse effects.
The IRA represents a critical juncture in healthcare policy. As researchers, we must collaborate to understand its effects fully. We are therefore calling for high-quality research papers, analytic reports, reviews, and commentaries on the IRA and its impact, whether intended or unintended. Areas of particular interest span the two key provisions of the IRA (i.e., Medicare Drug Price Negotiation & Affordability Reforms to the Medicare Part D Drug Benefit) discussed in more detail in our upcoming Editorial, as well as the following example questions:
Impact on Innovation: 1) What impact is this law having on pre-clinical investments, including VC investments? 2) Are there leading indicators being observed today that call into question the accuracy of the Congressional Budget Office (CBO) forecast? 3) Small molecules constitute a significant portion of drug development. How does the IRA impact research and innovation in this area? Are there potential disincentives for small molecule drug discovery due to pricing constraints? 4) How are the IRA reforms likely to impact generic and biosimilar entry? 5) What is the impact on life cycle management and label/indication expansion?
Impact on Beneficiary Out-of-Pocket (OOP) Costs: 1) What formulary status changes and other formulary management tool changes can we observe before and after MFPs are in place for the first 10 selected drugs? 2) How are patient OOP costs changing for the selected drugs as well as the classes affected? 3) What are the list-net price changes for drugs within the same classes as the selected drugs?
Impact on Beneficiary Access: 1) How are insurance plans responding to their increased liability – in terms of prior authorizations, step-edits, formulary generosity, tiering, premium changes, plan offerings? 2) How does the IRA affect patient access to essential medications? Are there unintended consequences that hinder access for vulnerable populations?
Assessment of IRA Implementation by CMS: 1) Once the methodology for Maximum Fair Prices (MFPs) is published by CMS for the first 10 selected drugs, what can we glean from the approach used to arrive at the MFPs? 2) Did patient clinical benefit factors appear to play more or less of a role?
The Collection is open for submission to everyone. Be part of the conversation and help us spotlight recent research findings, viewpoints, analyses, and insights by publishing your work with us.
Publishing Model: Hybrid
Deadline: Ongoing

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in