The social little brain: Understanding the role of the cerebellum in social disorders
Published in Neuroscience
The cerebellum is typically associated with motor function, but work from our lab and others has shown that the cerebellum should not be discounted in guidance of complex social behaviors. The cerebellum has vast connections throughout the brain and these neural circuits are emerging as critical drivers of sociability. In particular, we asked whether cerebellar dysfunction during sensitive developmental windows in early-life may drive cortical development and maturation. Development of these pathways may influence how social an organism is as an adult and even how they parent their own offspring. We are very interested in the cerebellar nuclei, as these output “hubs” of the cerebellar cortex are thought to have long-range influence on distal brain regions. In particular, the dentate or lateral cerebellar nuclei (LCN) has been implicated in neurodevelopmental disorders, including autism and attention-deficit disorder, which have a tendency to be co-diagnosed.
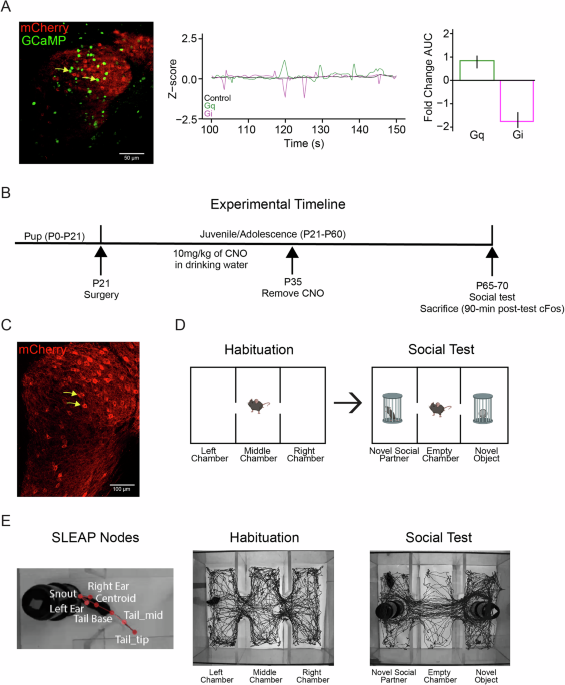
To investigate these pathways, we used a viral-based strategy called Designer Receptors Exclusively Activated by Designer Drugs (DREADDS), to increase or decrease cerebellar nuclei neural firing in a mouse model. To simulate an early-life disruption to typical cerebellar activity, we perturbed the cells in the LCN at postnatal day 21, which roughly corresponds to birth or early infancy in humans. While we analyzed males and females, male animals demonstrated larger changes in sociability and circuit-level dysfunction. This sex-selective vulnerability aligns with longstanding clinical observations that males are disproportionately affected by neurodevelopmental and neuropsychiatric conditions involving motivation, reward, affect, and cognitive control. The biological basis for this difference remains elusive.
To understand why social behavior was altered, we explored connections with the LCN to the prefrontal cortex. We analyzed multiple regions of the prefrontal cortex, but found only specific changes to neural firing and structure within the anterior cingulate cortex (ACC). Interestingly, a subtle developmental disruption to the LCN was enough to reorganize activity across three major brain regions known for motivation, reinforcement learning, and cognitive control: the ventral tegmental area (VTA), nucleus accumbens (NA), and anterior cingulate cortex (ACC). These are not places traditionally associated with cerebellar function, yet activity shifted in a circuit-level way that traced back to the cerebellum. Not only were these changes in LCN-VTA-NA-ACC connections functional, but we observed structural remodeling of neurons in the ACC suggesting that the cerebellum shapes these cortical circuits in development.
Our findings show that cerebellar output pathways may be one of the upstream developmental nodes that diverge between sexes. In addition, this study suggests that the cerebellum may orchestrate changes in dopaminergic, limbic, and prefrontal brain circuits. We uncovered a mechanistic bridge between the cerebellum and the neural systems governing motivation and decision-making. To completely understand the brain, we need to study it in its entirety and not discount the cerebellum.
Why this matters
For a long time, the cerebellum was thought to be mainly involved in movement, including balance and coordination. Our research shows that the cerebellum also plays an important role in shaping social behavior, motivation, and decision-making. We found that small disruptions to cerebellar activity during a critical early-life period can cause lasting changes in brain circuits that navigate social interactions and reward. These changes were particularly strong in males, which may help explain higher male prevalence in certain neurodevelopmental conditions (e.g., autism spectrum disorder). Importantly, the cerebellum influenced brain areas not usually linked to it, including regions involved in learning from rewards to making choices. This work suggests that early brain development is more interconnected than previously thought, and that disruptions in one area can ripple across the brain. Understanding these connections could help guide earlier detection and better treatments for neurodevelopmental disorders that impact the lives of millions of families.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in