
Red Velvet Coffee & Chocolate Cookies
The story of two young scientists began in the summer of 2019. Hui Ye, visiting Professor Lei Wang at UCSF—a pioneer in genetic code expansion (GCE) research—met Nanxi Wang, who was pursuing her postdoctoral studies in Wang’s lab. One sunny afternoon, they sat together at SPARK Social Food Truck Park, just steps away from the lab, savoring red velvet lattes and chocolate cookies. That seemingly simple moment, filled with warm sunlight and the tempting aroma of coffee, was the start of something special—a journey of friendship and collaboration.

Tools for Lactylation Detection and Modification
By 2020, both Nanxi and Hui had embarked on their own research paths in the field of protein post-translational modifications (PTMs), lactylation as a start. Hui focused on developing mass spectrometry-based methods for lactylation detection, while Nanxi worked on site-specific lactylation modification using GCE. Recognizing their shared interests and complementary expertises, they decided to join forces to build a convenient workflow that integrates proteomics to identify lactylated sites and GCE for the expression of site-specifically lactylated proteins in living cells, and an integrated functional analysis (IFA) platform to evaluate the biological consequences following lactylation.
Their first collaborative effort bore fruit after two years of dedicated work. In their publication in Nature Methods (https://www.nature.com/articles/s41592-022-01523-1) , they revealed a conserved set of lysine lactylation sites in the human proteome by leveraging cyclic immonium ions derived from lactylated lysine residues by searching public unmodified proteome datasets. Additionally, they explored the regulatory mechanism of "end-product inhibition" mediated by lactylation on the target protein ALDOA using purified proteins in vitro.
Pioneering New Directions in Lactylation Research
Building on this success, Nanxi and Hui tackled an even more ambitious challenge. In their recent publication in Nature Communications (https://www.nature.com/articles/s41467-024-55165-2) , they introduced simpler and more accessible genetic code expansion tools designed to study lysine lactylation and its biological significance in mammalian cells. Their efforts led to the engineering of highly efficient and selective enzymes capable of site-specific lactylation in both bacterial and mammalian cells. This advancement significantly enhanced the second module of their workflow, making it more versatile and streamlined.
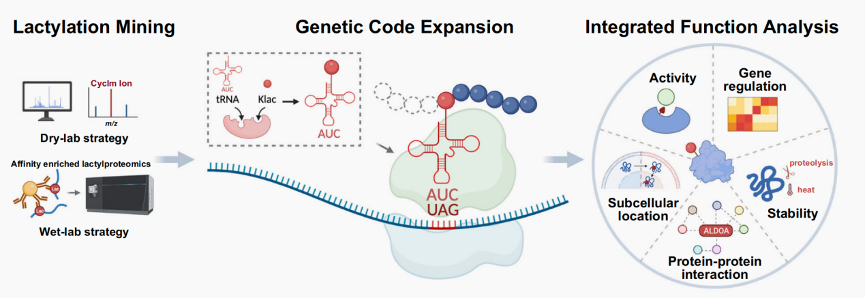
The upgraded workflow not only simplifies the study of lysine lactylation in living cells but also opens up new avenues for investigating the biological roles of this emerging PTM at virtually any lactylation site. Using this innovation, they revealed the gain-of-function effects of ALDOA lactylation in mammalian cells, shedding light on how this modification influences protein stability, enzymatic activity, and nuclear translocation.
Their discoveries underscore the importance of lactylation in cellular regulation, but they also raise intriguing questions about its broader implications. How might lactylation influence other metabolic pathways? How significantly can single-residue lactylation modulate protein interactions? Could it play a role in disease states, such as cancer or inflammation? These are questions that Nanxi and Hui are eager to address in future studies.
A Shared Vision for the Future
What began as a simple meeting over coffee and cookies has grown into a meaningful collaboration that combines proteomics, metabolomics, molecular biology, and chemical biology. Nanxi and Hui are driven by a shared desire to develop technologies that can contribute to scientific understanding and support the broader research community, with a particular focus on emerging PTMs like lactylation.
Looking forward, they hope their research—using mass spectrometry to identify novel PTMs and genetic code expansion (GCE) to incorporate them into proteins—will provide a useful framework for functional PTM studies. Through their work, they aim to shed light on new biological mechanisms and contribute to potential therapeutic developments, guided by a deep respect for collaboration, curiosity, and the joy of discovery.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in