Thermo-responsive compartmentalized systems inspired by stress granules
Published in Bioengineering & Biotechnology and Materials

My lab mates and I, led by Prof. Ho Cheung Shum at the University of Hong Kong, have developed a thermo-responsive compartmentalized system inspired by stress granules. Stress granules would assemble rapidly to resist stress and maintain homeostasis by storing biomolecules and mediating metabolism via interception of signal molecules under external stimulation, such as heat shock. The unique advantages offered by membraneless organelles towards signal transduction, biomolecule storage, and gene expression attracts drastic interest from the science community in designing artificial counterparts.
In this study, we develop a thermo-responsive ATPS (TR-ATPS) that allows regulating two-level compartmentalization through two-step phase separation. The TR-ATPS comprising thermo-responsive poly (N-isopropylacrylamide) (PNIPAM) and dextran (DEX) displays sensitive phase transition behaviors in response to temperature variations. In a PNIPAM (5 wt%)/DEX (5 wt%) solution, liquid membraneless compartments enriched in PNIPAM separate from the continuous DEX-rich phase at 25 ℃. When the temperature is increased to 35 ℃, the size of PNIPAM compartments decreases sharply from 56-145 μm to 14-60 μm, accompanied by the generation of small second-level compartments (~1 μm) at the interface (Fig. 1).
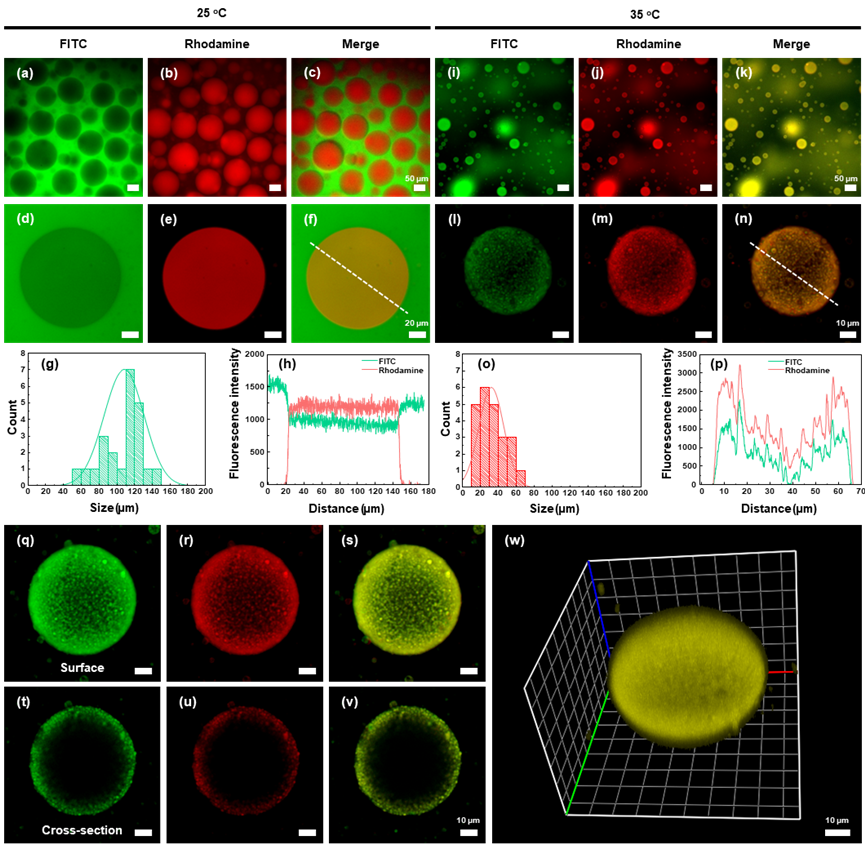
The second-step phase separation of the TR-ATPS is thermally reversible, according to the reconfiguration of PNIPAM chains between hydrophilic coils to hydrophobic globules. The transition temperature of the TR-ATPS is around 31 ℃, close to the human body temperature, rendering the system suitable for investigating biochemical reactions in vitro. As a demonstration, we introduce glucose oxidase and horseradish peroxidase into the TR-ATPS and observe their colocalization in the colloidosome-like compartments when the temperature is increased to 35 ℃. The enrichment of enzymes in the small second-level compartments with large surface area accelerates the tandem enzymatic reaction efficiency by approximately 7-fold (Fig. 2).
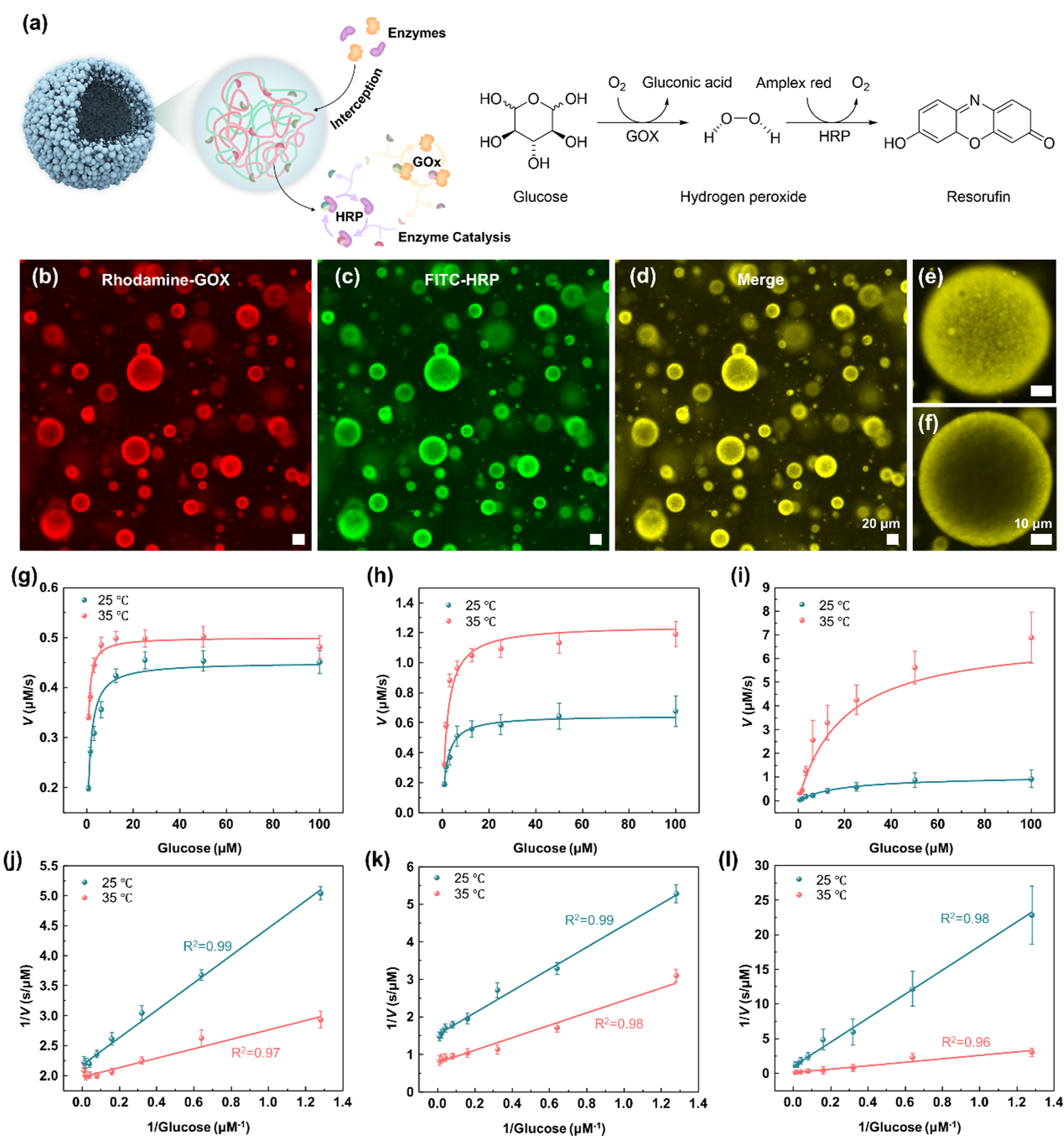
The TR-ATPSs capable of programing the distribution of biomolecules have the potential to enrich target biomarkers, facilitating rapid and point-of-care diagnosis. The TR-ATPSs display thermally reconfigurable structures between emulsion droplets and colloidosomes in a controllable manner. The unique feature has rarely been reported in artificial systems. ATPSs form two immiscible liquid phases that can be easily separated based on their different densities, allowing for efficient recovery of the encapsulated biomolecules. Besides, combining the advantages of aqueous two-phase systems with thermally reconfigurable features presents a unique system design that has not been extensively explored. This approach offers opportunities for the development of innovative enzyme-based technologies and applications. Compared with previously reported ATPSs compartmentalization methods, such as osmosis-driven and evaporation-driven phase separation of a single phase ATPS droplet, the compartmentalization of TR-ATPSs controlled by varying the temperature is more reconfigurable and controllable. In particular, unlike the inactivation of biomolecules caused by the complete evaporation of single-phase ATPS droplets or high osmolarity conditions, the temperature-regulated feature renders TR-ATPSs higher biocompatibility under biomolecules-involved scenarios by keeping them in mild aqueous environment.
The concept of TR-ATPS should be applicable to ATPSs containing other thermo-responsive polymers, which display similar LCST-type phase transition with PNIPAM, such as poly (N, N-diethylacrylamide) (PDEA). Moreover, the approach illustrated by TR-ATPSs may be extended to the design of other types of stimuli-responsive ATPSs, including pH-responsive ATPSs, light-responsive ATPSs, and multiple-responsive ATPSs. For instance, selecting azobenzene-contained polymers as one component can prepare light-responsive ATPSs, in which the compartmentalization can be reversibly triggered by light irradiation. For ATPSs composed of two or more types of stimuli-responsive polymers, the compartmentalization can be regulated by different physicochemical signals, creating opportunities in synthetic biology and biochemical engineering.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in