Thiostrepton alleviates experimental colitis by promoting RORγt ubiquitination and modulating dysbiosis
Published in Immunology
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic relapsing inflammatory disorder caused by an inappropriate immune response. Due to more than half of all IBD patients do not achieve 1-year clinical remission and thus experience disease progression, it is imperative to develop new drugs for the treatment of IBD.
As clinical physician of the Gastroenterology department, we have never ceased our ongoing research on gastrointestinal diseases and positively curing the patients. In our research group, we have been committed to dissecting the pathogenic mechanism and seeking efficient drugs for IBD treatment. It must be acknowledged that the precise pathogenesis of IBD has not been well disclosed, but multiple lines of evidence suggest a crucial role of specific members of the microbiota in driving intestinal inflammation. Thus, we sought to find therapeutic antibiotic to treat the disease. Thiostrepton (TST), an FDA-approved natural cyclic oligopeptide antibiotic, exhibits multiple pharmacological properties, including antimicrobial, anti-cancer, and even anti-inflammatory properties1-4, but the efficacy of TST in the context of colonic inflammation has rarely been reported.
In this study, the colitis model was constructed with dextran sulfate sodium (DSS), then followed with TST administration, 7 days later, the TST treated mice exhibited significantly attenuated disease phenotypes. Additionally, TST treatment also limited the development of T-cell transfer colitis. Based on those findings, our team members were determined to probe the mechanism of how TST alleviates colitis. The overproduction of proinflammatory cytokines has been directly implicated in the exacerbation of IBD, we did find that TST treatment decreased the production of inflammatory cytokine IL-17A and the frequency of IL-17A-producing cells. The transcription factor RORγt regulates Il17a transcription, as expected, TST treatment also resulted in corresponding reductions in RORγt-positive IL-17A-expressing cells.
After confirming TST treatment could reduce RORγt expression, we began to investigate the exact pattern. TST was considered as inhibitor of transcription factor FOXM1 due to the ability to reduce FOXM1 expression, but FOXM1 could not regulate Rorc expression at transcription level. We next found MG132 treatment attenuated the reduction in RORγt protein expression caused by TST. By unbiased screening of E3 ligases (Itch and UBR5) and deubiquitinases (USP4 and USP17), Itch was confirmed to coimmunoprecipitated with RORγt, and we understand more solid results should be done in Itch-/- cells and Itch-/- transgenic mice. Thus, Dr. Ya Luo constructed Itch-/- 293 T cells by CRISPR/Cas9 system through conducting PCR detection experiment on nearly 1200 transduced cells. Besides, all our team members participated in phenotyping and breeding the Itch-/- and Il17a-EGFP transgenic mice. Of note, the highly conserved PPXY motif of RORγt could potentially interact with the WW domains of Itch5, thus TST may possibly function as a molecular glue degrader to facilitate the assembly of RORγt and Itch. By utilizing molecular docking, Dr. Ya Luo and Dr. Xianglian Zhang did find TST could dock to and bind well to the binding interface of the Itch-RORγt complex.
In addition, antibiotics can disrupt the gut microbiota, which in turn can affect the outcome of immune responses. By constant discussing with team members, we chose to clear gut microbiota. In our hands, after gut microbiota depletion, the protective effect of TST against DSS-induced colitis was partially impaired, but the TST-mediated frequency reduction in RORγt-expressing CD45+ cells was not prevented. The findings proved that inhibition of RORγt by TST occurs independently of the gut microbiota. Next, Dr. Ya Luo and Dr. Yuan Luo performed fecal microbiota transplantation (FMT) assay to confirm whether TST affects the ability of the gut microbiota to control intestinal inflammation. Indeed, the feces from TST-treated mice (with or without DSS administration) could alleviate DSS-induced colitis, these results indicated that gut microbiota shaped by TST conferred protection against DSS-induced colitis.
As to figure out how TST affected gut microbiota to relieve intestinal inflammation, we performed 16S rRNA gene amplicon sequencing and metagenome sequencing. The measurement of Chao1 and Shannon indices revealed that TST treatment reduced alpha diversity, reflecting its potent antimicrobial activity, and the beta diversity analysis indicated that gut microbiota shaped by TST possessed a distinct community composition from those in control group. Next, we assessed the landscape of gut microbiota and found that Bacteroidetes was the most predominant phylum in TST-treated group compared to control group. And at genus level, TST-treated group displayed different biological compositions to the control group. Furthermore, we analyzed the taxa by linear discriminant analysis (LDA) effect size (LEfSe) (LDA score >4) and found that Prevotella and Parabacteroides were more abundant in TST-treated group. Considering Short-chain fatty acids (SCFAs) are crucial in the maintenance of intestinal homeostasis, we quantified and found acetic acid, propionic acid, butyric acid, valeric acid and caproic acid levels were significantly increased with TST treatment. Collectively, TST improves the ability of the gut microbiota to control colitis.
Many findings of this study were interesting to us. On the one hand, TST exerts its direct immunomodulatory effects to suppress IL-17A production by targeting RORγt for ubiquitination and degradation. On the other hand, TST exerts its antimicrobial effects to ameliorate colitis by modulating gut microbiota dysbiosis. Therefore, we demonstrated, for the first time, TST plays a dual role in protecting mice from experimental colitis (Figure 1). These new insights hopefully help to understand better about the pathogenesis of colitis and screen for therapeutic drugs for colitis. Our next plan is to determine whether TST is a molecular glue degrader to facilitate the assembly of RORγt and Itch.
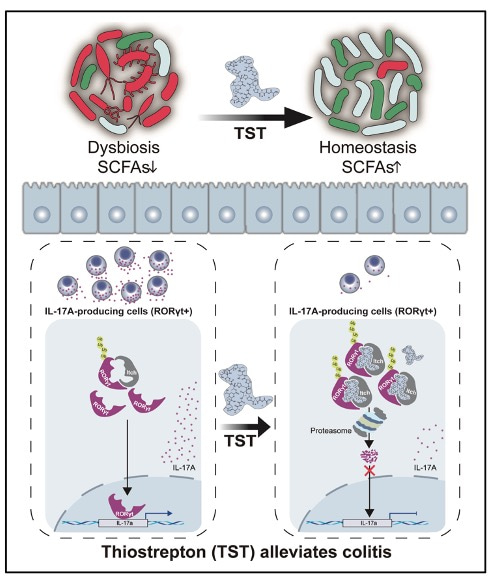
Figure 1. Graphical abstract. Working model of TST-mediated reduction in IL-17A producers and modulation of gut microbiota dysbiosis to prevent colitis.
We sincerely thank every fruitful discussion with Prof. Lilin Ye and Prof. Jihang Zhang. Besides, the comments from expert reviewers were crucial to us to improve the quality of the study. Of course, in the process of this research, all our team members have done well and made every effort to overcome all challenges.
References
1 Hegde, N. S., Sanders, D. A., Rodriguez, R. & Balasubramanian, S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem 3, 725-731, doi:10.1038/nchem.1114 (2011).
2 Lai, C. Y. et al. Identification of Thiostrepton as a Novel Inhibitor for Psoriasis-like Inflammation Induced by TLR7-9. J Immunol 195, 3912-3921, doi:10.4049/jimmunol.1500194 (2015).
3 Kim, T. H. et al. Thiostrepton: A Novel Therapeutic Drug Candidate for Mycobacterium abscessus Infection. Molecules 24, doi:10.3390/molecules24244511 (2019).
4 Walter, J. D., Hunter, M., Cobb, M., Traeger, G. & Spiegel, P. C. Thiostrepton inhibits stable 70S ribosome binding and ribosome-dependent GTPase activation of elongation factor G and elongation factor 4. Nucleic Acids Res 40, 360-370, doi:10.1093/nar/gkr623 (2012).
5 Kathania, M. et al. Itch inhibits IL-17-mediated colon inflammation and tumorigenesis by ROR-gammat ubiquitination. Nat Immunol 17, 997-1004, doi:10.1038/ni.3488 (2016).
Follow the Topic
-
Cellular & Molecular Immunology

A monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, covering both basic immunology research and clinical applications.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcement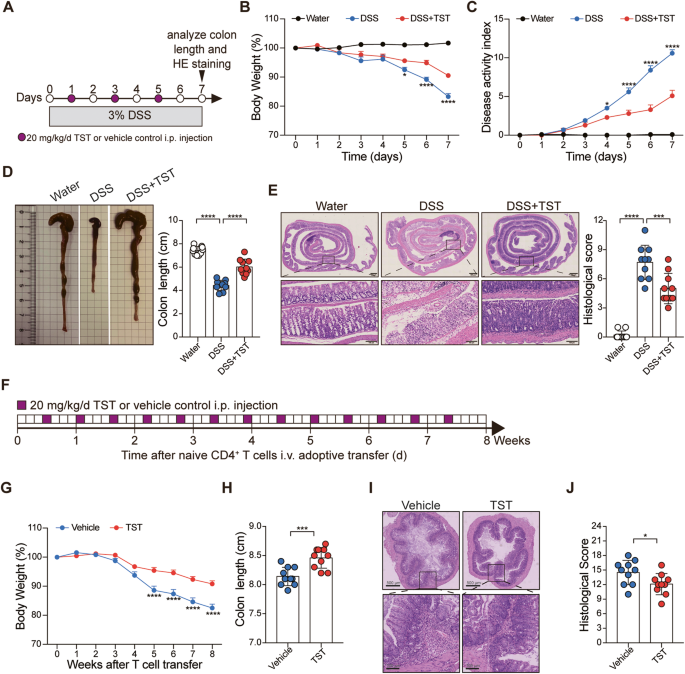
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in