To die or not to die: that is a Dickkopf question
Published in Neuroscience

The enteric nervous system (ENS) represents the intrinsic innervation of the gastrointestinal tract acting partly autonomously and independent of central nervous system processing. Comprised of cells, that arise from the embryonic neural crest, the ENS is organized in two major ganglionated plexus, namely the myenteric and submucosal plexus, localized within the gastrointestinal wall. ENS reflex pathways control intestinal motility, local blood flow, transmucosal movement of fluids, or secretion of gut hormones1,2.
Development after birth
At birth, the ENS is still immature and underlies considerable refinements to exert its proper function. These refinements include changes in the neurochemical coding and synaptic wiring of submucosal and myenteric neurons3. Interestingly, a notable number of ENS-cells undergo enteric neurogenesis within the first postnatal days in rodents4. However, enteric neurogenesis in the adult ENS is controversially discussed and arguably a rare event in vivo5,6. Although many in vitro studies demonstrated a high regernative potential of postnatal ENS-progenitor cells of various rodent species and human patients7-9, only a few models of chemical denervation10 and pathological settings like colitis11 resulted in regenerative neurogenesis of the ENS in vivo. This intriguing discrepancy between the proliferative potential of ENS-progenitor cells in vivo and in vitro suggest that ENS-homeostasis might be strictly regulated by cell-cell-communication systems to maintain the steady state of the adult ENS. These cell-cell-communication systems and their influence on ENS-homeostasis in health and disease drew increasing attention over the last years.
Role of Wnt-signaling in the ENS
In this context, the Wnt-signaling pathway plays an essential role in the regulation of proliferation and differentiation of neural stem and progenitor cells, and has been studied extensively as a key regulator of adult stem cell homeostasis in different metazoan tissues12. With respect to the ENS, Wnt-signaling induces and specifies neural crest formation and is involved in regulating enteric neural cell migration, guidance, and growth of enteric neuronal projections in the developing gut13,14. Our group and others have shown that canonical Wnt-signaling increases the proliferative capacity of postnatal ENS-progenitors from rodent models and human infants in vitro and successively leads to a higher yield of newly generated neurons15,16. Due to its pivotal role in regulating proliferation and differentiation in various organs, Wnt-signaling is tightly regulated. A prominent group of Wnt/β-catenin-antagonists, are Dickkopf proteins, which are an evolutionary conserved gene family of four glycoproteins (DKK1-4)17. Recent evidence emerged, that DKK1 is involved in a variety of cellular/context-dependent functions, such as regulating cell proliferation and differentiation processes18-20, cell survival and programmed cell death21. In the present study, we hypothesized that DKK1 could function as a negative regulator on the proliferation of ENS-progenitors in the postnatal gut of mice and human infants. Our results indicated that DKK1 indeed influenced ENS-progenitors, however, surprisingly not as initially expected.
To die or not to die: that is a Dickkopf question
To evaluate, the role of DKK1 in the postnatal gut, we first analysed the expression pattern of DKK-ligands/receptors in small and large intestine of mice and humans with in situ hybridisation and immunhistochemistry. DKK-ligands and receptors were expressed within submucosal and myenteric ganglia of the murine and human intestine. Intringuingly, we detected DKK-mRNA and proteins at particularly high level within the neuropil of the ganglia. Thus, the involvement of neuronal and glial processes in intercellular DKK-signalling is very compelling and demands future investigations.
Giving the in vivo expression, we evaluated the cell biological function of DKK1 on the in vitro model system of postnatal ENS-progenitor cells. For this purpose, we isolated ENS-progenitor cells from the muscular layer of the intestine of mice as well as from paediatric gut samples. Cultivation of murine and human ENS-progenitor cells under proliferation conditions resulted in 3-dimensional spheroids, termed enterospheres, which also comprised non-neuronal cells of the Tunica muscularis, such as smooth muscle cells or fibroblasts. We consider this an advantage over purified neurospheres from transgenic mice as it resembles the environment of the in vivo situation more accurately. Beyond that, we conducted DKK1-stimulation experiments in spheroid cultures of Wnt1-Cre reporter mice. In these mice, neural cells express the red fluorescent protein tdTomato, which can be employed to generate purified neural cultures from Tunica muscularis using FACS sorting, thus enabling conclusions on the direct interaction of ENS cells.
Molecular biological evaluation revealed that postnatal ENS-progenitors are equipped with receptors and signaling cascade components essential for pharmacological probing. But, surprisingly, on a cellular level, DKK1-stimulation led to a significant and profound increase in the proliferation of the P75+ neural cell population and in the size of murine spheroids in ex vivo cultures, forcing us to reject our initial hypothesis. Astonishingly, however, the pro-proliferative effect of DKK1 on P75+ neural cell population did not lead to an increase in the yield of newly-generated enteric neurons and glial cells. An effect, we were able to reproduce in human ENS-progenitor cells as well, thereby adding clinical relevance to our findings and indicating that this mechanism is conserved across different mammal species.
Moreover, several studies have shown, that DKK1 is involved in cell-cycle control mediating apoptotic-mechanisms arguably by Caspase-3 activity21. To further address the role of DKK1-mediated apoptosis, cell death assays and live-monitoring-Caspase-3/7 activity assays showed, that the profound increase in ENS-progenitor cell proliferation was paralleled by an increased cell-death in the P75+ neural cell population by a Caspase-3/7-dependent mechanism. Yet, once we blocked apoptosis by applying a pan-Caspase inhibitor, DKK1 stimulation markedly increased enteric neurogenesis and gliogenesis in ENS-progenitors. This indicates that the cell-death-induction by DKK1 can be rescued without interrupting the pro-proliferative effect of this Wnt-antagonist.
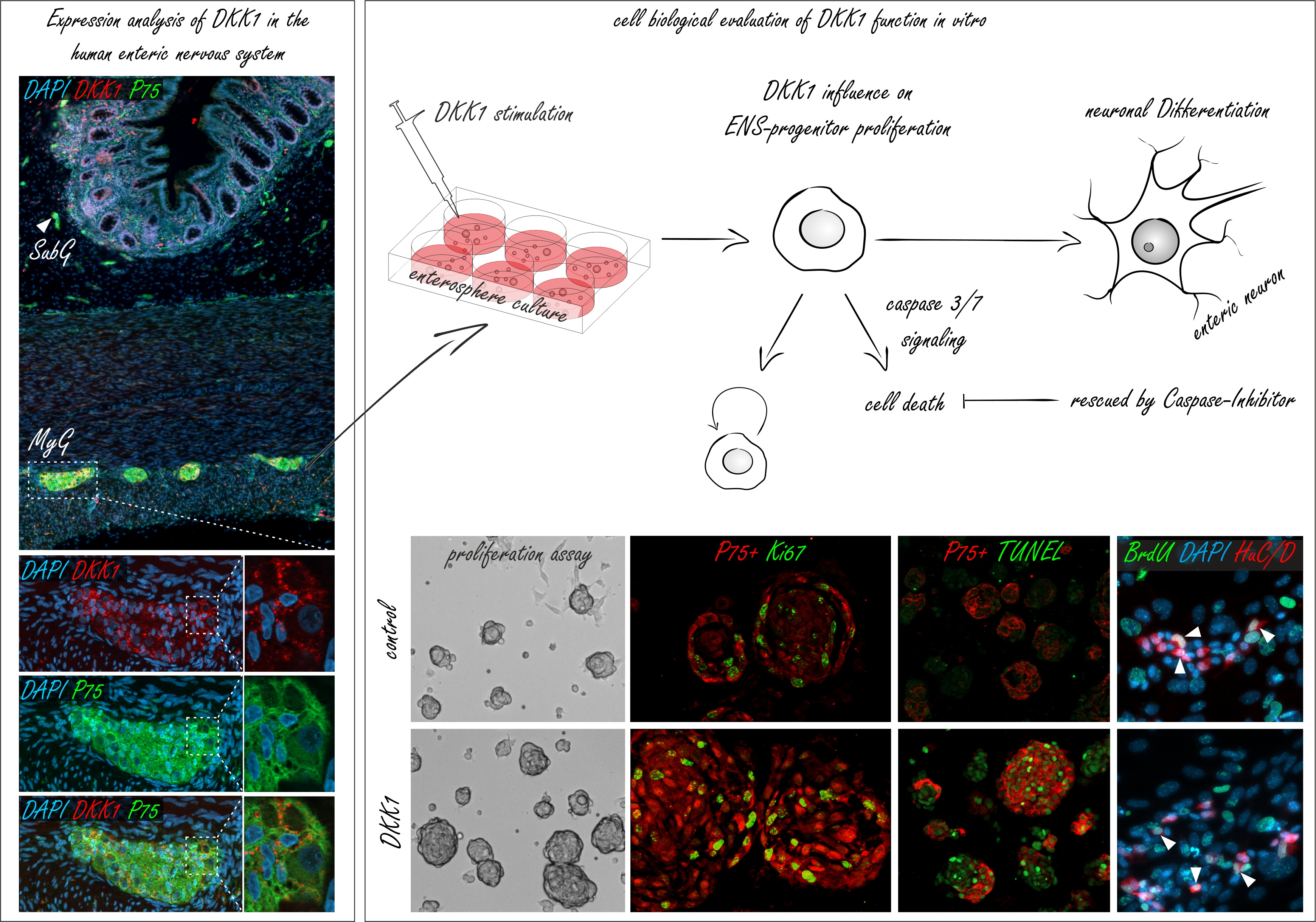
| Immunohistochemical experiments on intact human gut tissues (left-side), showed that Dickkopf1 (DKK1) is co-expressed with P75 positive neurites in submucosal (SubG, arrow head) and myenteric ganglia (MyG, white box) of the human intestine. Thus, we probed the cell biological function of DKK1 on mouse and human ENS-progenitors in vitro (right-side). Molecular biological evaluation revealed that our in vitro model system of postnatal ENS-progenitors is equipped with receptors and signaling cascade components essential for pharmacological probing. Surprisingly, DKK1-stimulation led to a significant and profound increase in the proliferation of the P75+ neural cell population (P75+Ki67+) and in the size of murine spheroids in in-vitro cultures. Astonishingly, the pro-proliferative effect of DKK1 on P75+ neural cell population did not lead to an increase in the yield of newly-generated enteric neurons (BrdU+HuC/D+). The profound increase in ENS-progenitor cell proliferation was paralleled by an increased cell-death of the P75+ neural cell population (P75+TUNEL+) by a Caspase-3/7-dependent mechanism. Yet, once we blocked apoptosis by applying a pan-Caspase inhibitor, DKK1 stimulation markedly increased enteric neurogenesis in ENS-progenitors. |
Concluding remarks
Together with previous work15-16, our study suggests, that ENS cells are functionally equipped with the suitable receptor/ligand repertoire in vivo, and that ENS-progenitors in vitro are tightly regulated by a multidimensional regulatory network of Wnt-signaling components.
We like to point out, that the data presented does not assess the physiological function of DKK1 on postnatal ENS-progenitor cells in vivo. As Wnt-signaling serves as a multitude of vital functions in various tissues, manipulations including for instance DKK1-knockouts are detrimental and not viable until the desired postnatal stage22. However, entero- and neurospheres are one possible in vitro model system to study for instance the impact of molecular signaling in neuro/gliogenesis and differentiation of postnatal ENS-progenitors.
Our findings suggest, that DKK1 is a strong, ambivalent regulator of the ENS-progenitor cell pool in mice and humans. These results are fundamental steps to reshaping our understanding of the homeostasis of the ENS in health and disease. Thereby, the developmental importance of DKK1 for orchestrating an appropriate amount of proliferating ENS-progenitor cells should be further investigated, thus helping to further understand basic pathomechanisms of enteric neuropathies including hypo- and aganglionosis-disorders with a high degree of patient suffering.
Scharr, M., Scherer, S., Hirt, B. et al. Dickkopf1 induces enteric neurogenesis and gliogenesis in vitro if apoptosis is evaded. Commun Biol 6, 808 (2023). https://doi.org/10.1038/s42003-023-05072-x
References
- Nagy N, Goldstein AM. Enteric nervous system development: A crest cell's journey from neural tube to colon. Semin Cell Dev Biol 2017;66:94-106.
- Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med 2009;13:1193-210.
- Parathan P, Wang Y, Leembruggen AJL, et al. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Developmental Biology 2020;458:75-87.
- Bergner AJ, Stamp LA, Gonsalvez DG, et al. Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J Comp Neurol 2014;522:514-27.
- Boesmans W, Nash A, Tasnády KR, et al. Development, Diversity, and Neurogenic Capacity of Enteric Glia. Front Cell Dev Biol 2021;9:775102.
- Virtanen H, Garton DR, Andressoo J-O. Myenteric Neurons Do Not Replicate in Small Intestine Under Normal Physiological Conditions in Adult Mouse. Cellular and Molecular Gastroenterology and Hepatology 2022;14:27-34.
- Metzger M, Bareiss PM, Danker T, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology 2009;137:2063-2073.e4.
- Metzger M, Caldwell C, Barlow AJ, et al. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology 2009;136:2214-25.e1-3.
- Almond S, Lindley RM, Kenny SE, et al. Characterisation and transplantation of enteric nervous system progenitor cells. Gut 2007;56:489-96.
- Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 2011;121:3412-24.
- Belkind-Gerson J, Graham HK, Reynolds J, et al. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Scientific Reports 2017;7:2525.
- Clevers H, Loh KM, Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014;346:1248012.
- Sasselli V, Boesmans W, Vanden Berghe P, et al. Planar cell polarity genes control the connectivity of enteric neurons. J Clin Invest 2013;123:1763-72.
- Nagy N, Kovacs T, Stavely R, et al. Avian ceca are indispensable for hindgut enteric nervous system development. Development 2021;148.
- Neckel PH, Scharr M, Seid K, et al. Wnt Receptor Frizzled-4 as a Marker for Isolation of Enteric Neural Progenitors in Human Children. Cells 2019;8:792.
- Zhang Y, Seid K, Obermayr F, et al. Activation of Wnt Signaling Increases Numbers of Enteric Neurons Derived From Neonatal Mouse and Human Progenitor Cells. Gastroenterology 2017;153:154-165.e9.
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006;25:7469-81.
- Verani R, Cappuccio I, Spinsanti P, et al. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem 2007;100:242-50.
- Kunke D, Bryja V, Mygland L, et al. Inhibition of canonical Wnt signaling promotes gliogenesis in P0-NSCs. Biochem Biophys Res Commun 2009;386:628-33.
- Ribeiro D, Ellwanger K, Glagow D, et al. Dkk1 regulates ventral midbrain dopaminergic differentiation and morphogenesis. PLoS One 2011;6:e15786.
- Causeret F, Sumia I, Pierani A. Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner. Cell Death Differ 2016;23:323-32.
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001;1:423-34.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in