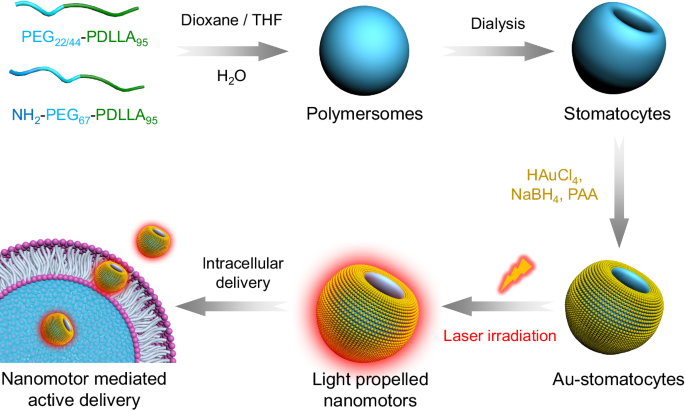
In recent years, self-propelled autonomous micro- and nano-motors have been employed as a novel drug delivery system in the biomedical field due to their capability of converting various sources of energy into mechanical movement for active cargo delivery into hard-to-reach tissues. A recent contribution of our group was the development of a nanomotor chassis composed of bowl-shaped polymersomes, also called stomatocytes1. To meet the requirements for biomedical applications, biodegradable stomatocytes were synthesised from block polymers of poly(ethylene glycol)-b-poly (D, L-lactide) (PEG-PDLLA)2. Block copolymer self-assembly into spherical vesicles and subsequent dialysis-mediated shape transformation provided us the desired asymmetric morphology, which guarantees directional movement when propelled by external stimuli. Additionally, the streamlined shape of the stomatocytes was also beneficial for their movement. To construct stomatocyte-based nanomotors, we equipped these stomatocytes for example with manganese dioxide particles in the cavity3, or with a gold hemispherical shell on the stomatocyte surface4.
Obviously, nanomotor velocity is one of the important parameters affecting their function. Nanomotors with a very high velocity could lead to better tissue penetration, more efficient intracellular drug delivery, and enhanced accumulation in the target area. Light-propelled nanomotors display high velocity and have controllable behavior. This can for example be achieved by decorating the motor chassis with a gold nanolayer, which, due to the plasmonic effect can turn light into heat. The heat gradient then inuces motility. However, until now it was proven difficult to construct PEG-PDLLA stomatocyte with such a gold layer. This stomatocyte collapsed easily when sputter coating was applied. Furthermore, most Au-based structures employed were larger than 10 nm, whereas a decrease in size could be highly beneficial for the efficiency of energy conversion and for many applications in nanomedicine. Hence, it is a challenge to construct light-propelled nanomotors based on soft materials. In this investigation we have found a solution for this challenge.
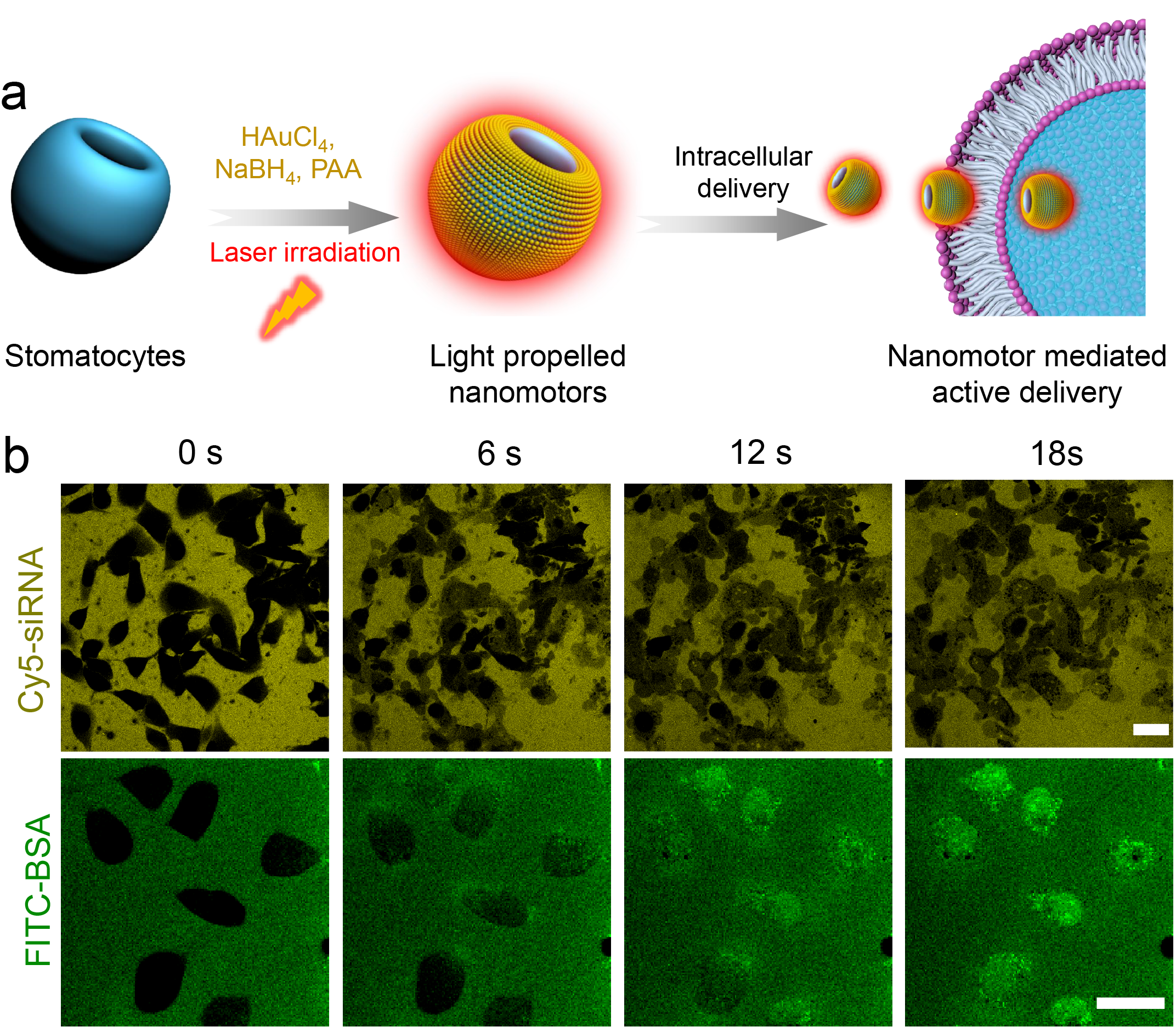
Fig. 1 a) Design of hybrid photoactivated nanomotors. b) Intracellular delivery of Cy5-siRNA and FITC-BSA mediated by Au-stomatocyte nanomotors upon 800 nm laser irradiation.
The first step of this work involved the synthesis of biodegradable amphiphilic block copolymers of PEG-PDLLA, which upon self-assembly and subsequent dialysis against salt solution resulted in bowl-shaped stomatocytes. To overcome the obstacles of sputter coating or the decoration with larger Au components, 5 nm Au NPs were coated on the outer surface of stomatocytes using the in situ growth method by non-covalent conjugation. The prepared hybrid stomatocytes displayed several intriguing properties. For instance, the inherent asymmetric morphology of Au-stomatocytes enabled the hybrid particles to harness near-infrared (NIR) irradiation and generate a thermal gradient, which is capable of triggering autonomous motion. Furthermore, the generated plasmonic heat also resulted in photothermal therapy. By analyzing their motion, we found that light propelled Au-stomatocyte nanomotors displayed excellent motility in both pure water and biological medium (PBS and DMEM), with maximum velocities between 105 and 125 μm s-1. To the best of our knowledge, our light-activated stomatocyte-based nanomotors thereby outperform currently reported nanomotor systems. In order to explain this feature, the 3D morphology of the nanomotors was analyzed in detail by quantitative cryo-electron tomography (cryo-ET), which revealed that the bowl-shaped stomatocytes were endowed with an asymmetric arrangement of Au NPs along the z-axial direction, resulting in uneven distribution of plasmonic heating upon laser irradiation. Consequently, the Au-stomatocytes exhibited directional motion with high velocities. The motile features of the nanomotors were furthermore exploited to achieve translocation of the particles in mammalian cells via the temporary disruption of the cell membrane. When this process was performed in presence of biomolecules, these were also effectively taken up, without affecting cell viability.
This project was challenging but very rewarding. We collaborated with a multidisciplinary team with backgrounds in polymer chemistry, biomedical engineering, and physics, which allowed us to understand the nanomotor features from design to application and further reveal the movement mechanism. Although many nanomotors have been developed for the biomedical field, we are confident that our system will attract a broad readership and inspire researchers to design biodegradable soft-materials-based nanomotors and explore their biomedical applications. We sincerely hope that this system will contribute to the design of soft-materials-based nanomotor systems and foster collaboration with researchers in different fields to promote practical applications in the clinic.
To learn more about our work, please read our article "Ultrafast light-activated polymeric nanomotors" in Nature Communications (https://doi.org/10.1038/s41467-024-49217-w).
Reference
- Wilson, D. A., Nolte, R. J. M. & van Hest, J. C. M. Autonomous movement of platinum-loaded stomatocytes. Chem. 4, 268-274 (2012).
- Pijpers, I. A. B., Abdelmohsen, L. K. E. A., Williams, D. S. & van Hest, J. C. M. Morphology Under Control: Engineering Biodegradable Stomatocytes. ACS Macro Lett. 6, 1217-1222 (2017).
- Pijpers, I. A. B. et al. Hybrid Biodegradable Nanomotors through Compartmentalized Synthesis. Nano Lett. 20, 4472-4480 (2020).
- Shao, J. X. et al. Twin-Engine Janus Supramolecular Nanomotors with Counterbalanced Motion. Am. Chem. Soc. 144, 11246-11252 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in