Uncovering a Hidden Mucosal Fungus Ally in the Fight Against Intestinal Inflammation
Published in Microbiology, General & Internal Medicine, and Anatomy & Physiology

Crohn's disease (CD) is a relentless inflammatory bowel disease that affects millions worldwide, causing chronic pain, fatigue, and life-altering disruptions. While we've long known that the gut microbiome—the trillions of bacteria, viruses, and fungi living in our intestines—plays a role in CD, most research has focused on the compositionality and functionality of luminal bacteria in IBD. But what if the real story lies deeper—what about the gut fungi? Can they cling to the intestinal mucosal lining and thereby exert a role? — These questions drove our study, entitled "Gut mucosal mycobiome profiling in Crohn's disease uncovers an AMP-mediated anti-inflammatory effect of Cladosporium sphaerospermum [https://www.nature.com/articles/s42255-025-01420-9]", now published in Nature Microbiology.
While gut bacteria have long dominated microbiome research, the fungal fraction is often overlooked. Fungi are not as flashy as bacteria due to their low abundance in the gut, and studying them is notoriously tricky. But in CD, where inflammation ravages the small intestine's mucosa, we suspected that fungi might harbour untapped biological secrets, potentially residing in overlooked niches of the gut. This paper represents a multi-year odyssey involving trans-domain explorations from multi-cohort humans to in vitro and animal studies.

The Spark: From Faeces to Mucosa
At the very start, we lamented how faecal studies dominate microbiome research—easy to collect, but perhaps missing the action at the mucosal interface, where microbes directly interact with our immune system. CD primarily hits the terminal ileum, so why not go straight to the source?
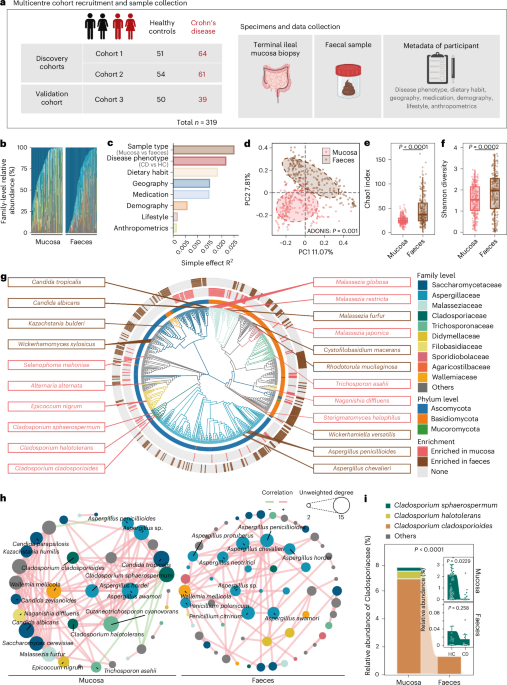
However, collecting ileal biopsies via endoscopy was no small feat; it's invasive, requiring patient consent and precise timing to avoid contamination. Ultimately, we enrolled 319 participants (164 CD patients and 155 healthy controls) from three geographically diverse cohorts to ensure robustness. We profiled not just the mycobiome (using ITS sequencing) but also the bacteriome (16S and metagenomics), metabolome (UPLC-MS/MS and GC-MS).
The Discovery: A Mucosal Mystery Unveiled
Early analyses revealed a bombshell: the mucosal mycobiome is worlds apart from the faecal one. Mucosa teems with Cladosporium and Malassezia species, forming a tight-knit ecological network, while faeces favour Candida and Aspergillus. Diversity is lower in mucosa, but interactions are stronger—like a small, elite club versus a bustling party.
In CD patients, the mucosal mycobiome was profoundly distorted. Using machine learning (random forest classifiers), we pinpointed 37 signature fungal species differentiating CD from healthy controls, with an AUC of 0.97—better than the performance of bacterial profiles! Among them, a star fungus, Cladosporium sphaerospermum, was depleted specifically in CD mucosa compared to HC mucosa, but unchanged between CD faeces and HC faeces. This mucosa-specific signal was an eureka moment. This fungus is hiding in plain sight, right where the inflammation hits hardest.
However, correlation isn't causation. We dove into metabolomics and found purine metabolites like AMP (adenosine 5'-monophosphate) depleted in CD faeces, strongly correlating with C. sphaerospermum abundance. Genome sequencing confirmed C. sphaerospermum harbours a full purine salvage pathway, and in vitro cultures showed it secretes AMP prolifically—outpacing 28 other gut commensal fungi we tested.
The Challenges: When Genetic Editing Hits a Wall
The biggest roadblock came when we tried to genetically edit C. sphaerospermum itself to prove AMP’s causal role. There were no validated plasmids available for this species, and standard transformation methods—electroporation, Agrobacterium-mediated delivery, protoplast transformation—simply failed to work. Our team spent three solid months troubleshooting every possible condition, but we could not achieve stable genetic modification in C. sphaerospermum.
Rather than give up on mechanistic causality, we pivoted strategically: we turned to Aspergillus niger, a phylogenetically related, genetically tractable fungus with well-established tools and naturally low AMP production. Ultimately, we successfully generated isogenic strains—one overexpressing AMP synthesis genes and one knocking them down. When we gavaged these engineered strains into DSS-colitis mice, only the AMP-overexpressing strain conferred protection—mirroring the effects of wild-type C. sphaerospermum and pure AMP supplementation. This workaround provided the rigorous causal evidence we needed and turned a frustrating dead end into a decisive breakthrough. Yet each obstacle sharpened the final story.
The Bigger Picture: From Bench to Bedside?
This study flips the script: fungi aren't just pathogens; C. sphaerospermum is a probiotic powerhouse, potentially harnessable for CD therapy. Unlike bacteria, which struggle to colonize, this mucosa-tropic fungus could deliver AMP locally, fortifying barriers without systemic side effects.
Implications ripple outward. There may be additional untapped salutary fungal species/strains in the gut that could protect against IBD and possibly other diseases? Overall, IBD patients deserve better options beyond immunosuppressants. If C. sphaerospermum inspires fungal-based therapies, we've cracked open a new frontier. Stay tuned—the gut mycobiome has more stories to tell.
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in