Uncovering a New Role for KSR1 in Hippo Signaling
Published in Cell & Molecular Biology
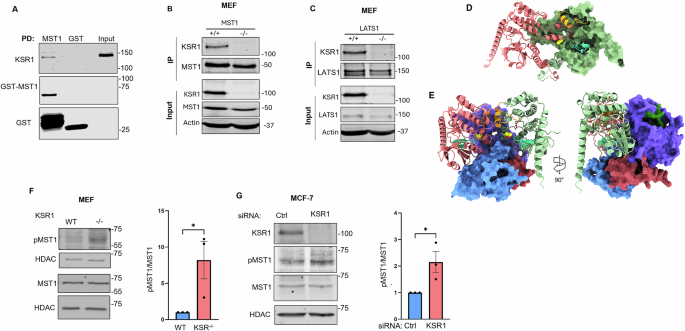
This project began with a simple curiosity: could a MAPK scaffold like KSR1 influence other major signaling pathways? Early experiments offered an unexpected clue—KSR1 consistently appeared in complexes with MST1, LATS1, and YAP. What seemed like a technical anomaly quickly became a turning point.
KSR1 was not just brushing against the Hippo pathway—it was organizing it.
As we followed this lead, diverse approaches—structural modeling, proteomics, imaging, and transcriptional analysis—converged on the same idea:
KSR1 functions as a scaffold for Hippo signaling, regulating YAP levels, nuclear localization, and mechanosensitive behavior through the RhoA–actin network.
One of the most striking findings was that external cues such as EGF could shift KSR1’s role between the MAPK and Hippo pathways, revealing a dynamic regulatory switch rather than a static scaffold.
Behind the scenes, this work required building new technical infrastructure and collaborating across structural biology, computation, and mechanobiology. Each piece brought clarity to a system that had been hiding in plain sight.
In the end, our study reframes KSR1 as a central coordinator of growth-control networks—crossing boundaries between mechanical cues, kinase cascades, and transcriptional programs.
Sometimes the most familiar proteins carry the most surprising stories; we just have to follow where the data lead.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in