Uncovering the intracellular target of candidalysin
Published in Microbiology and Biomedical Research
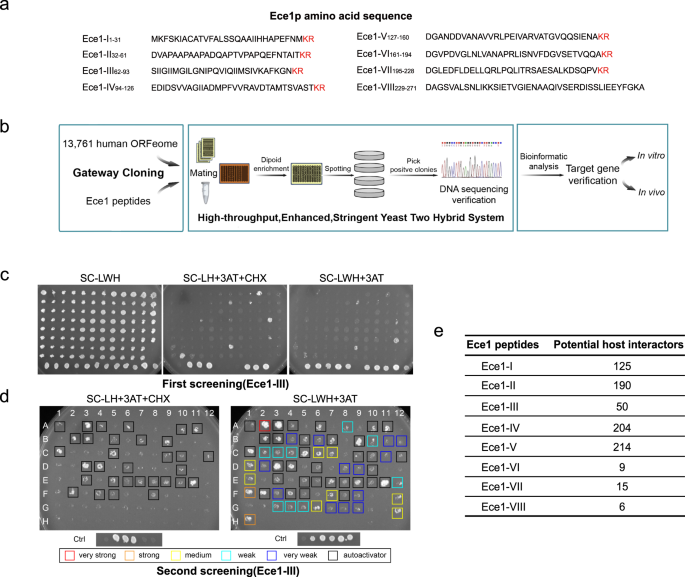
Candidalysin, as the first reported non-proteinase peptide toxin secreted by the major opportunistic human fungal pathogen-Candida albicans, has aroused our great interest. Previous work has shown that candidalysin damages the cell membrane to promote infection. However, its target remains unclear. Moreover, why does the fungi spend energy degrading the Ece1 protein into 8 different peptides with only one with toxin activity? Is there any specific target or host interactors for the other 7 different peptide? Is there any interaction between different peptides? This is the unanswered but charming question we are excited to explore.
We have constructed the interaction network of all eight Ece1 peptides with its human protein targets by a high-throughput enhanced yeast two-hybrid (HT-eY2H) screening, which not only revealing candidalysin-specific biological function clusters, but also shared and specific pathways among different peptides. More importantly, it provides a powerful method for future investigations on host-pathogen interactome. Mechanistically, we demonstrated that candidalysin hijacks the canonical DNA damage repair (DDR) pathway by targeting CCNH to promote fungal infection. Additionally, increasing evidence has demonstrated that CCNH expression is upregulated with development of several types of cancers, such as gastrointestinal stromal tumors (GIST), breast cancer, esophageal squamous cell carcinoma and brain tumors, suggesting the potential relationship between C. albicans infection and cancer progression.
It’s another attractive question whether the other seven Ece1 peptides secreted along with candidalysin have a particular role in fungi-host interaction. The interactome information we obtained from the Y2H screening provided us with certain clues about their biological processes each Ece1 peptide might participate in, such as the cell cycle and cell proliferation, cytoskeleton and cell connection, infection and immune regulation.
Surprisingly, we discovered the effect of candidalysin on olfaction that was suggested by functional enrichment, which provided a new theoretical basis for the complications related to olfactory damage in patients with fungal infections in clinic. However, we cannot exclude the possibility that the detrimental effects of candidalysin treatment on host olfactory function might also be due to the destruction of olfactory cells, considering the property of a cytolysin. The mysterious connection between fungal infections and olfaction awaits further investigation.
Together, our study not only provide the human interactors of Ece1-peptides including candidalysin which may serve as potential therapeutic targets, but also make it possible in the future to analyze the genome-wide interactome between the CANDIDA ORFeome and the Human ORFeome at the species level.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in