Understanding high pressure molecular hydrogen with a hierarchical machine-learned potential
Published in Chemistry
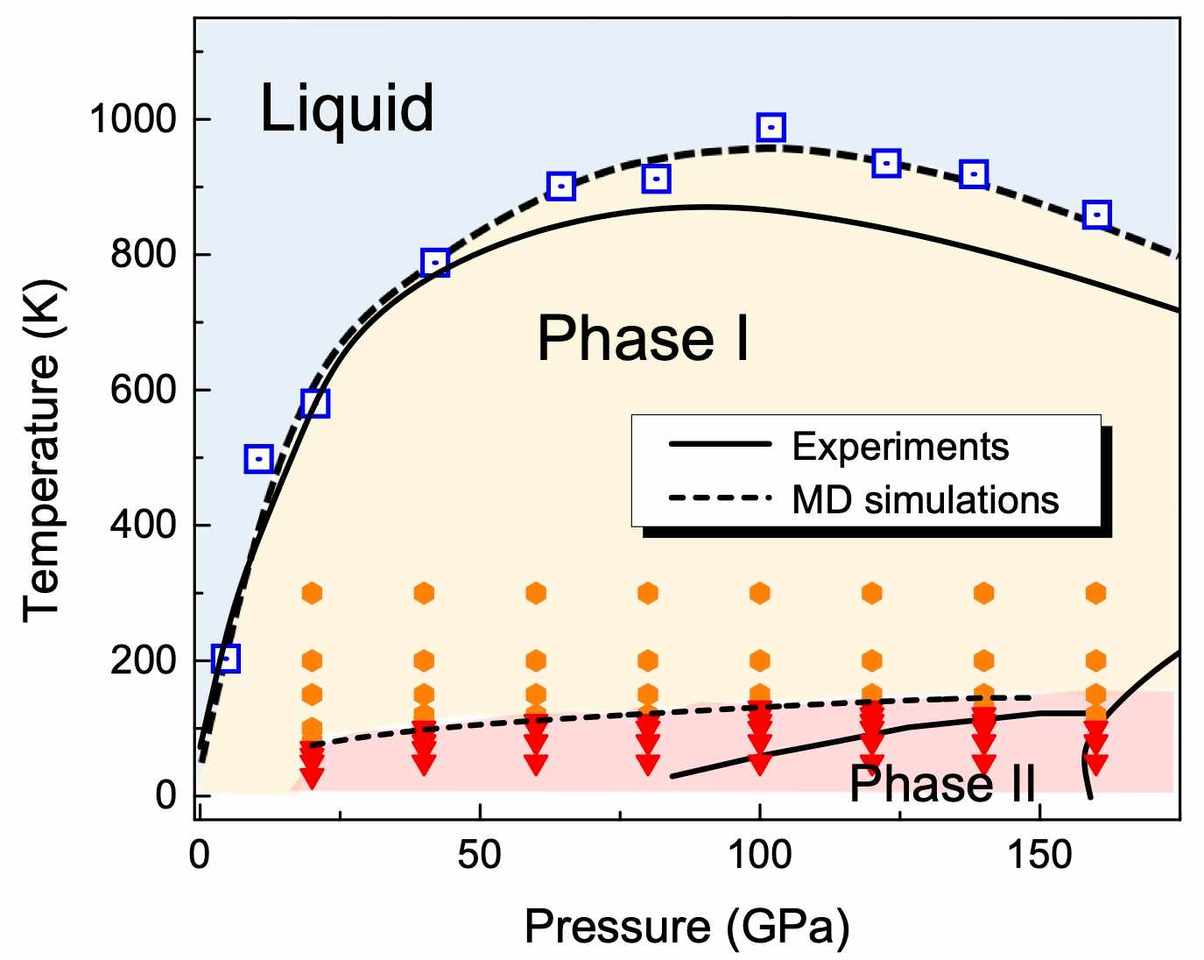
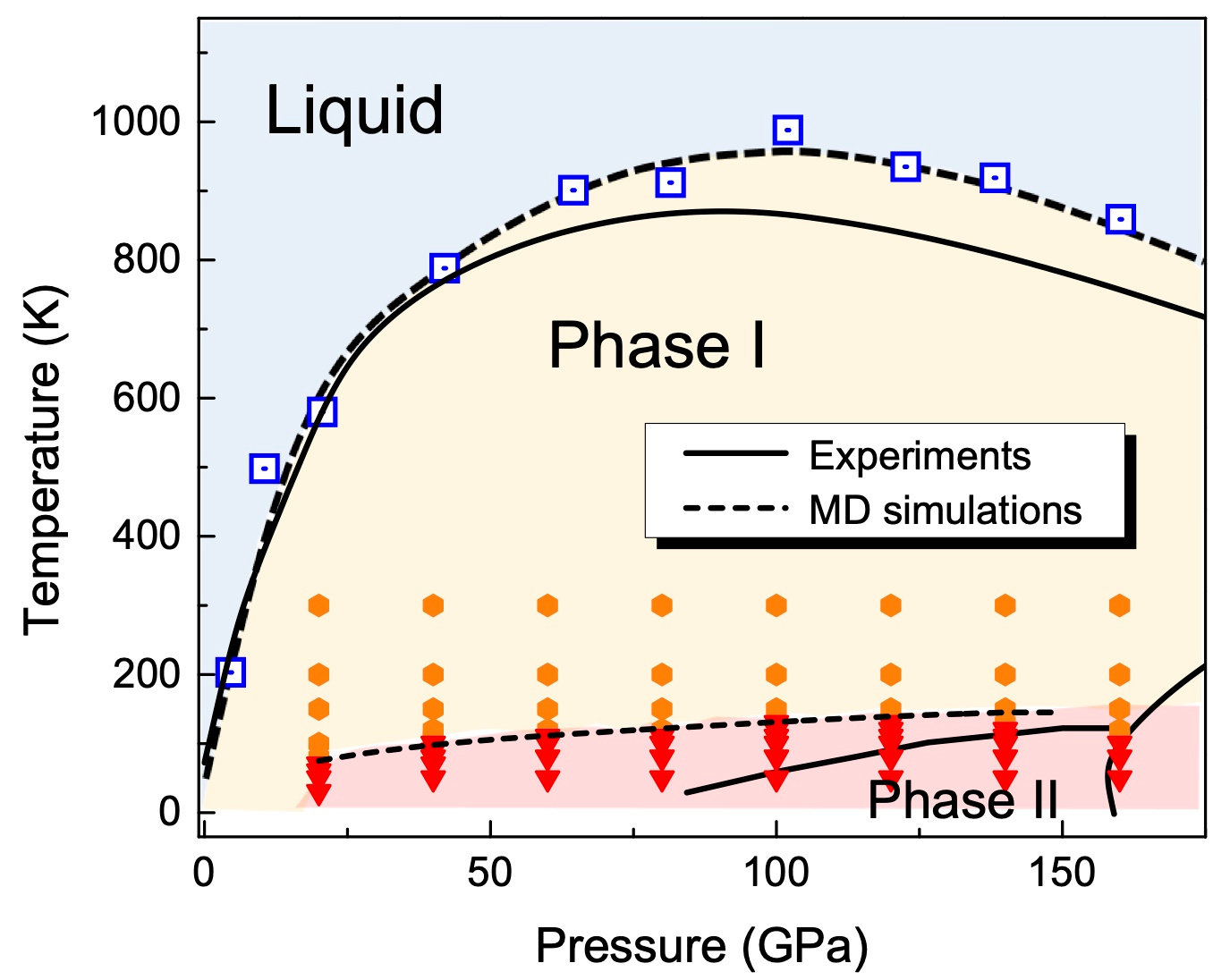
At very high pressure, hydrogen can be compressed into a solid or liquid phase, and these phases are weird. Because hydrogen is light, quantum mechanics are supposed to be important. But can quantum effects matter at at 1000K, the melt point? We wanted to investigate with a model where we can turn quantum mechanics on and off, to see if it makes a difference.
Everyone knows H2 is a diatomic molecule, but which way does it point? According to quantum mechanics, the lowest energy state, a rotor with zero angular momentum, has a quantum state with spherical symmetry (Y_00 spherical harmonic) - so it points every way at once. And, since the H2 molecule is a sphere, crystalline solid hydrogen "Phase I" is just a close packing of spheres. When you heat it up, it melts - no surprise there - but the melting line has a maximum temperature, beyond which the melt is denser than the close-packed solid. How could this be? It sounds like a quantum weirdness, but even in classical physics, H2 molecules can be spherical on average, if they are rotating, and so maybe this melting weirdness occurs classically too.
All this can be satisfactorily simulated with the workhorse of condensed-phase chemistry, the density functional theory. But to figure out why the liquid is denser, it would be necessary to simulate lots of liquid and solid arrangements, to see what's going on. So we built an interatomic forcefield which reproduces the DFT, and gathered lots of statistics.
Machine learning is the fancy new name for "fitting", but it is gratifying when in picks out real physics. We fitted all possible angular-dependent terms to the DFT data, and the fitted data showed that the dominant one is the quadrupole-quadrupole interaction. That fact won't surprise any chemist, but it was interesting that the algorithm could figure it out for itself.
For the liquid to be "denser than close packed" the molecules must be closer together, and this was the limit of previous analysis. A big surprise was the molecules are closer together but the atoms are not. This turns out to be because the lowest energy configuration for quadrupoles has molecular axis pointing at another molecule in a "T" shape, and this is common in the solid. In the liquid, "X"-shaped configurations where the atoms are further apart are favoured. With many atoms in three dimensions it gets more complicated, but we were able to tease a clear story out of the data.
The work was started by Dr Hongxiang Zong and Dr Heather Wiebe in Ackland's group in Edinburgh, and completed by Prof. Hongxiang Zong of Xi'an Jiaotong University and Prof. Heather Wiebe of Vancouver Island University.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in