Chemistry in a metallic environment
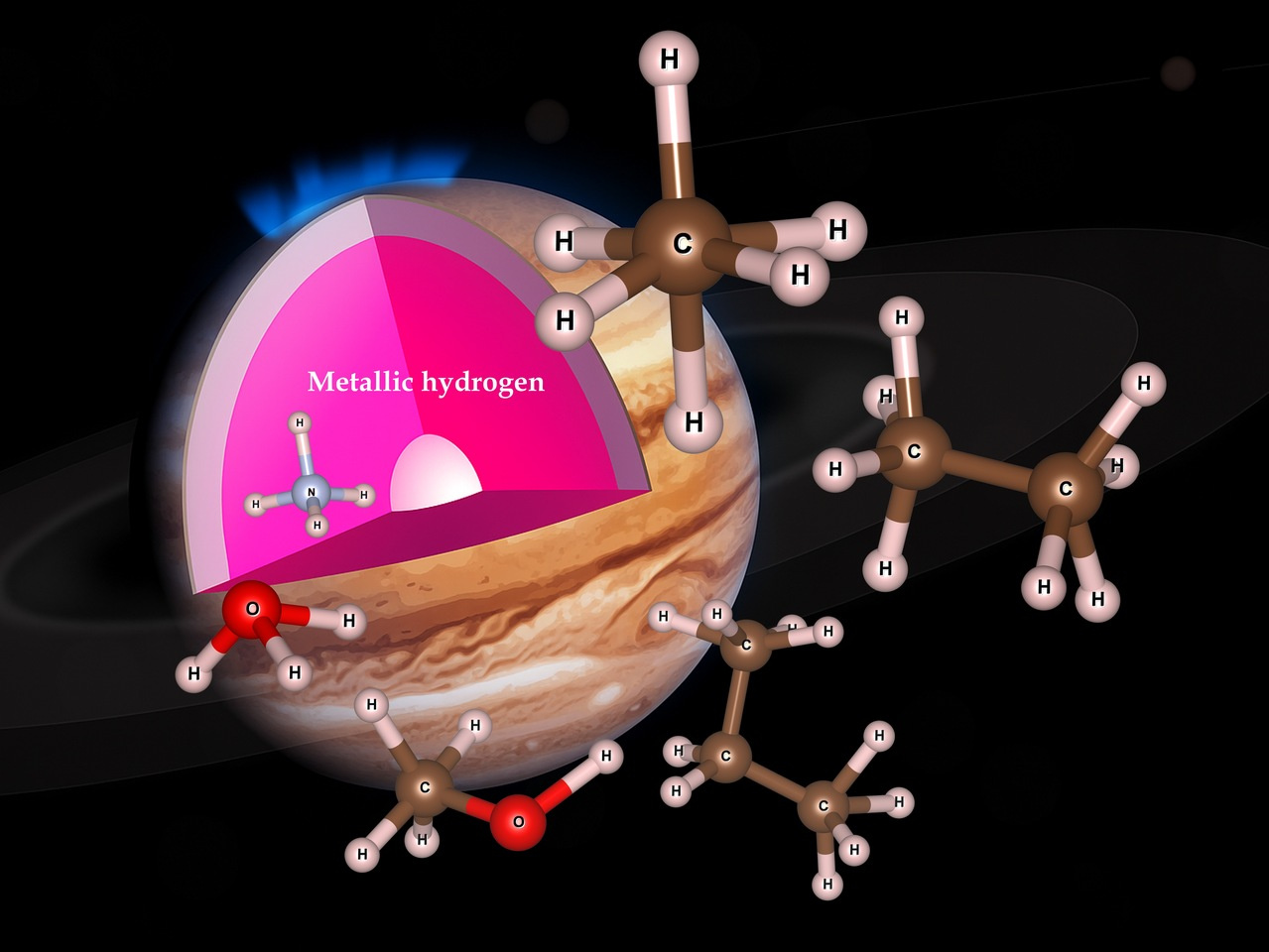
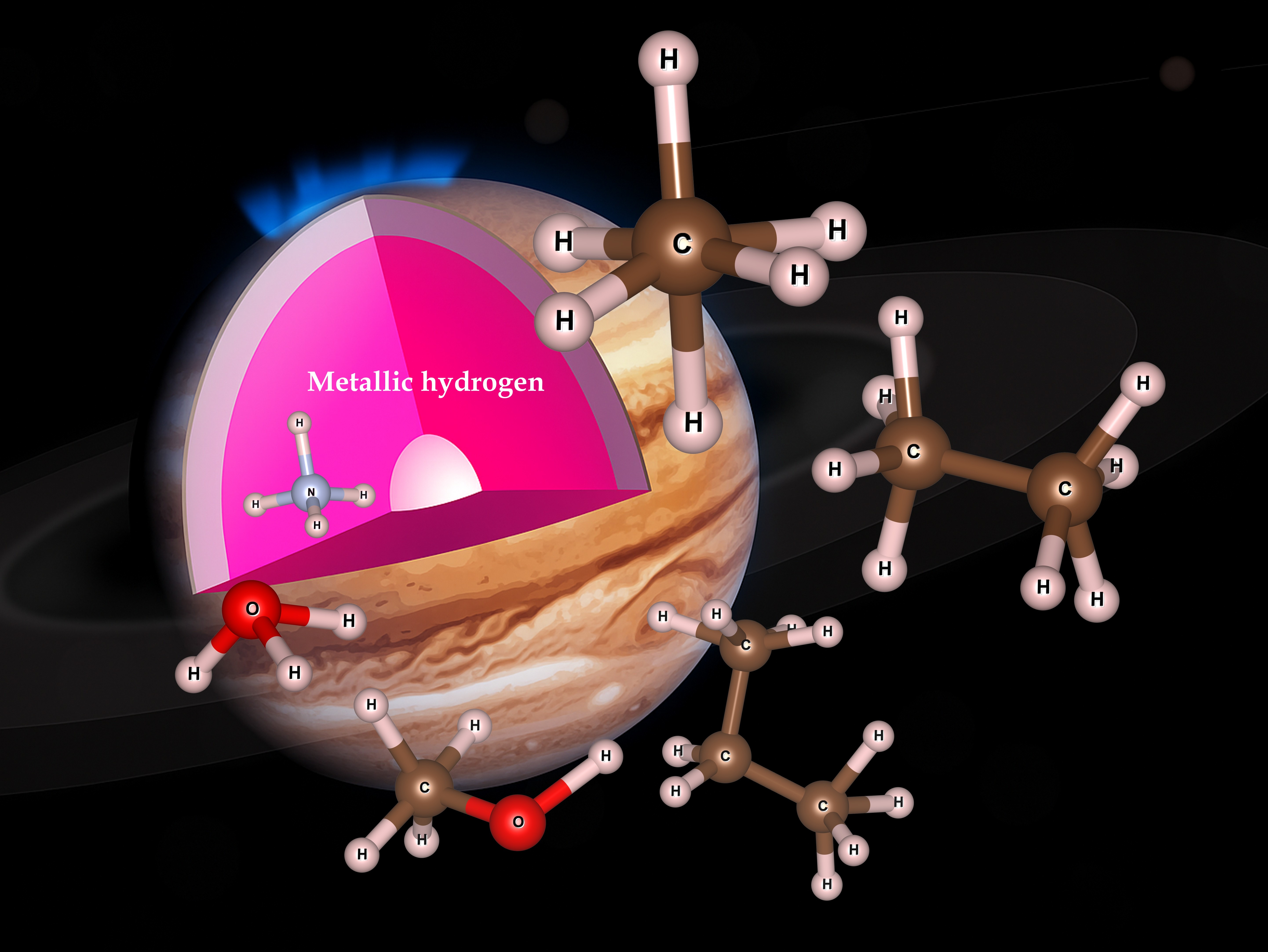
compositions form deep in planets
Chemistry usually takes place in aqueous solution, air or vacuum. Stable chemical compounds are usually limited by the number of valence electrons available for bond formation. But the most common environment in the solar system is high pressure metallic hydrogen, wherein extra electrons are available and charged molecules are also possible. So we can expect the rules of organic chemistry to be very different.
In Nature Communications, Jakkapat Seeyangnok and colleagues report the formation of small molecules in fluid metallic hydrogen. There are the counterparts of water, methane, ammonia, ethane and methanol, but with extra hydrogen atoms forming H3O, NH4, CH6, CH10, CH4OH. These compounds are known as hypermolecules, on account of these excess hydrogens. Analysis using standard chemistry techniques reveals these molecules to be covalently bonded. The work strongly suggests that unconventional organic chemistry with complex molecules can exist in a fluid metallic hydrogen environment, such as found in brown dwarfs. Quite how complex these hypermolecules can become remains to be ascertained.
In addition, we found that metallic hydrogen can act as a super-solvent for almost all elements, He and Ne being exceptions. This relates our study to the surprising discovery by the recent Juno mission that Jupiter appears to have no distinct rocky core: materials which could form rocks are simply dissolved in the metallic hydrogen.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in