Understanding the light induced hydrophilicity of metal-oxide thin films
Motivation for the work
Understanding the basic physical mechanisms for photocatalytic surface effects is important for applications such as water splitting, reduction of carbon dioxide to fuels using solar energy, decomposition of organic compounds, and light-induced hydrophilicity. The light-induced hydrophilicity is a property of materials, whereby their surfaces become more hydrophilic when exposed to ultraviolet (UV) light. For thin film metal oxides (MOx), such as titanium dioxide (TiO2) and zinc oxide (ZnO), the photocatalytic effects are very pronounced.
The significance of the bandgap
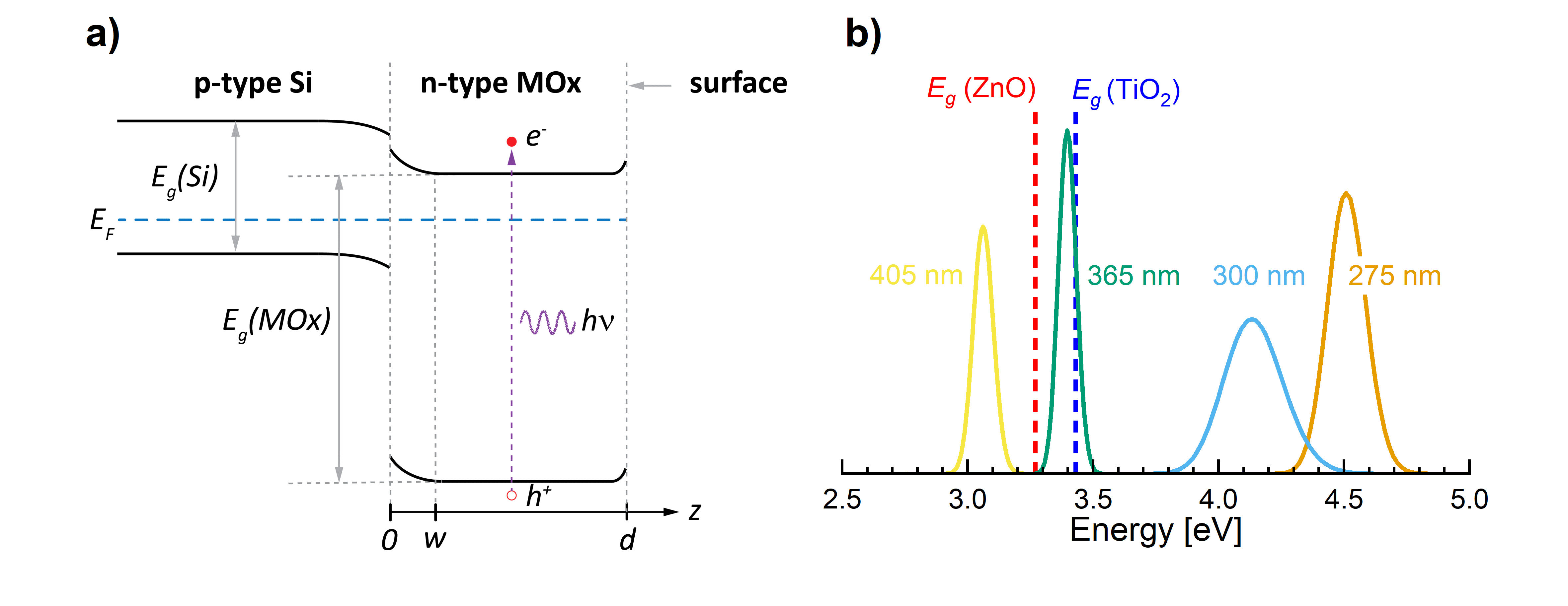
We readily observe the photocatalytic effects in TiO2 and ZnO because both materials have excitable surface groups, and simultaneously are semiconductors with bandgaps of order ~3.0-3.5 eV, which conveniently aligns with the photonic energy of UV light with a wavelength of 365 nm, the well-known i-line in the spectrum of commonly used mercury lamps. The link between the surface wetting properties and the bandgaps of the two materials is thus the chemical activation of the surface groups by photo generated electron-hole pairs. However, those electron-hole pairs can only be generated by light with a photonic energy that exceeds the bandgaps of the materials (Figure 1).
A model for the photocatalytic wettability in thin film metal oxides
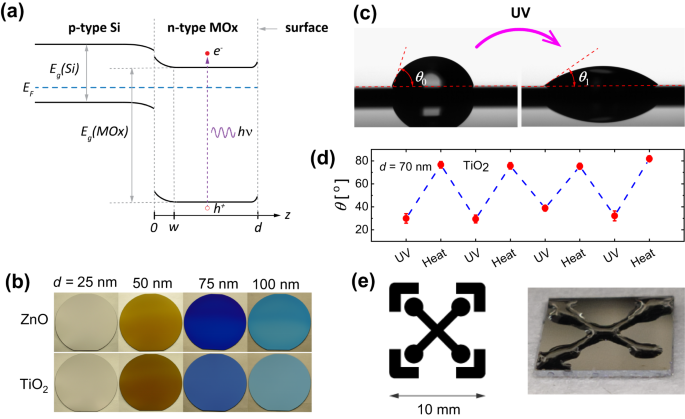
We propose a model for the involved mechanisms to induce hydrophilicity in thin films of TiO2 and ZnO. By “thin films”, we mean films that are considerably thinner than the UV wavelengths used for the surface activation, i.e. typically below 100 nm. For light incident on the MOx thin film, the photons may either be reflected, transmitted to the substrate, or absorbed in the MOx. The absorbed photons are those that generate the electron-hole pairs.
Several physical effects play a role in the process: 1) Fresnel reflections due to differences in refractive indices at both the air/MOx and the MOx/substrate interfaces play a role. 2) The fraction of absorbed photons to incident photons depend on the extinction coefficient at the photon wavelength in the thin film. 3) Multiple internal reflections at both interfaces of the MOx thin film result in a non-monotonic dependence of the absorptance (absorbed optical power to incident optical power) on MOx layer thickness. 4) While the electron-hole pairs are generated in the bulk of the thin film, they must travel to the surface by diffusion to activate the surface groups. However, electron-hole pairs generated too far from the surface may recombine before reaching the surface. Hence, also the diffusion length given by the diffusivity and the lifetime of the photogenerated carriers are important parameters. 5) Finally, the boundary condition at the MOx/substrate interface play an important role for the transport of the photogenerated carriers.
When reaching the surface, the electron-hole pairs engage in the surface reactions, where e.g. the holes cause oxidation of adsorbed water molecules, whereas electrons cause reduction of oxygen. The formed radicals may then engage in further reactions at the surface. In the beginning, the generated electron-hole pairs have plenty of surface groups to activate, however, by continuing the illumination, eventually all surface groups will become activated. Several competing chemical reactions with separate rate constants may take place when MOx are illuminated with UV light. However, in the proposed model, we assume that the reaction kinetics for the chemical reactions at the surface are first order in both the area concentration of inactivated surface groups and the photo generated excess carrier concentration at the surface. The activation rate (switching rate) of the surface states is found to be proportional to the illumination light intensity, the absorptance in the MOx layer, and the concentration of photo generated carriers at the surface.
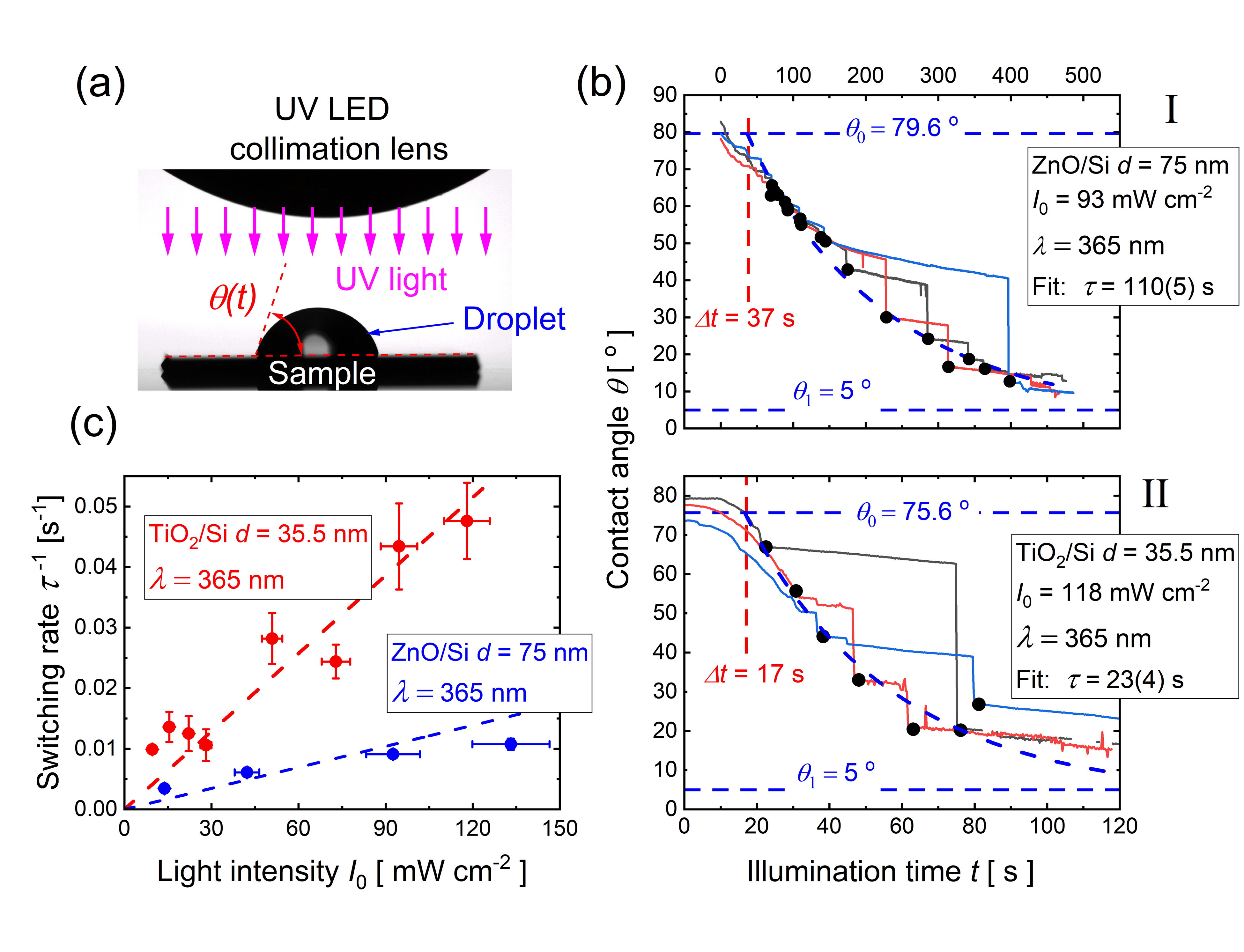
The activation rate for the surface groups obtained by contact angle measurements
The resulting photo-induced hydrophilicity is recorded by measuring the water contact angle at the surface during light illumination with a water droplet sitting on the surface. This contact angle can, however, be predicted in the proposed model by exploiting the classical Cassie-Baxter theory for chemically heterogeneous surfaces by attributing one surface chemistry to the non-activated surface groups, and the complementary surface chemistry to the activated surface groups.
The significance of the results
The most important finding we report is thus that the photocatalytic surface phenomena of metal oxides strongly depend on the optical constants (the refractive index and extinction coefficient) of the materials, and on the film thicknesses in a non-monotonic manner. In addition, our study reveals that the diffusion lengths of photo-generated carriers can be obtained from simple contact angle measurements. Those dependencies can be predicted within the framework of the presented physical model. Importantly, we can obtain design rules for the fastest possible switching surface.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in