Unexpected predominance of Archaea in a cool hyperacidic environment.
Published in Microbiology

From this very acidic site (pH 1.3-1.6) we have isolated and characterised a new archaeal genus and species, Cuniculiplasma divulgatum from a new family Cuniculiplasmataceae (order Thermoplasmatales). Comparative genomic analysis of Cuniculiplasma strains, which we isolated from Parys Mountain and Iberian pyritic belt site and a metagenomic assembly from a sibling organism from California pointed at a remarkable degree of conservation in their genomes (1,2).
Later, we described the association between C. divulgatum and its ectosymbiont (or ectoparasite) “Candidatus Mancarchaeum acidiphilum” (“Micrarchaeota”) and reported on the analysis and evolutionary patterns of this first ungapped genome in DPANN Superphylum (3). It was quite logical that our next step towards better understanding of microbial diversity of this hyperacidic ecosystem would be the assessment of the entire acidophilic microbiome and spatial distribution of microorganisms inhabiting water and sediment subsystems.
Shotgun metagenomics and SSU rRNA amplicon sequencing of DNA samples revealed that Euryarchaeota accounted for 67 % from the whole community, with the prevalence of Thermoplasmata (58%). Within Thermoplasmata, one particular group, “E-plasma”, without isolated representatives was in a majority (up to 43.5 % of total metagenomic reads) (Fig).
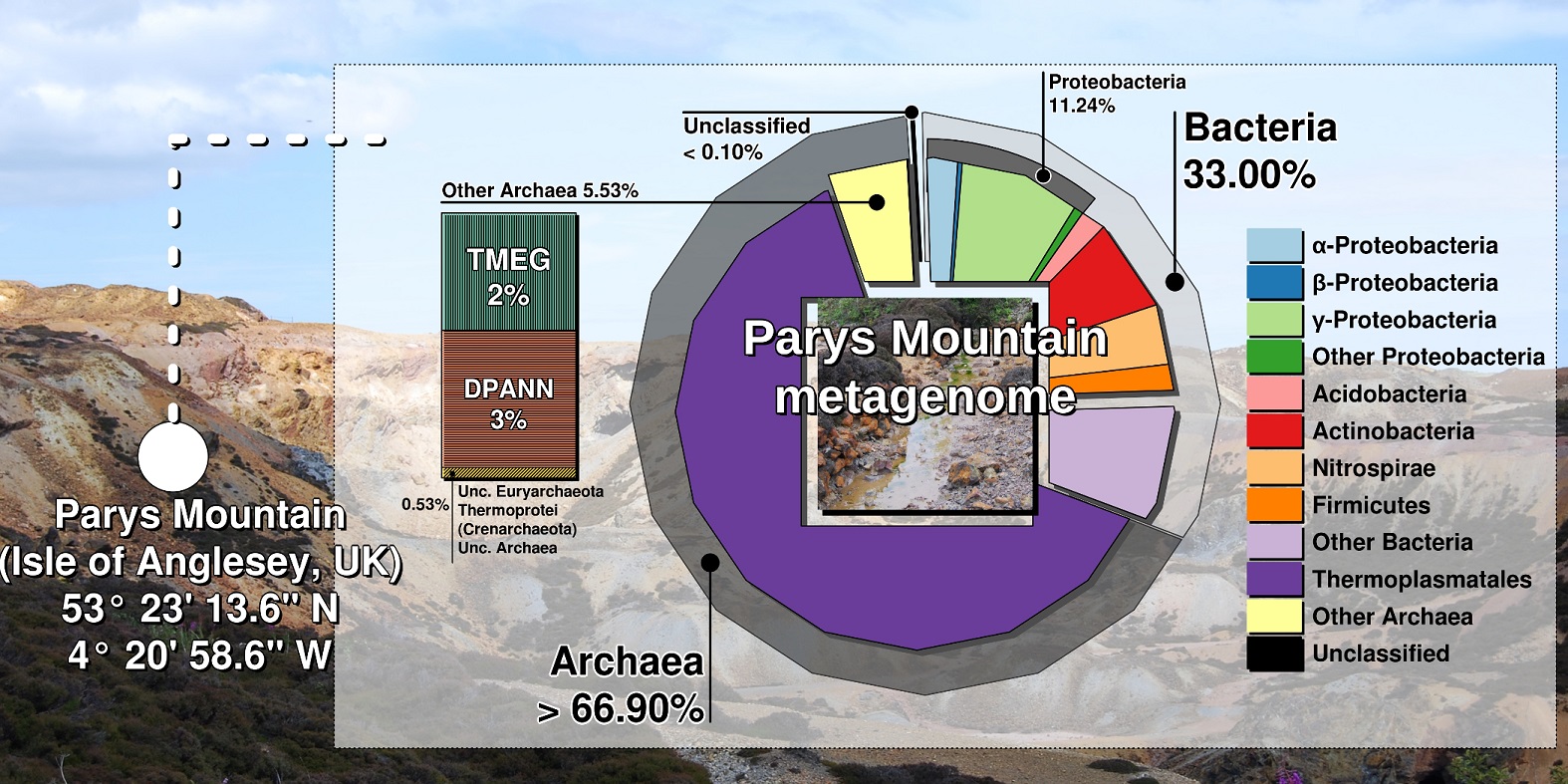
Among archaeal taxa, Cuniculiplasmataceae, “Candidatus Micrarchaeota” and “Terrestrial Miscellaneous Euryarchaeal Group” (TMEG) archaea were present in low numbers. TMEG archaea were earlier considered an “environmental clade” linked to Methanomassilicoccales-related archaea (4). Our global phylogenetic analysis of TMEG archaea showed strong clustering of Parys Mountain clonal variants with sequences detected in other acidic and moderately acidic places, pointing at the affiliation of this group with a new order within Thermoplasmata.
We also observed that this group of acidophilic organisms was often overlooked in acidic microbiomes. Highest numbers of archaea and in particular, Thermoplasmata were found in sediment samples, in contrast to the aqueous subsystem, where bacteria were in the majority. Furthermore, our analysis of microbial diversity in enrichment cultures pointed at the significance of sample pre-treatment, which could be used for isolation of cell wall-deficient archaea.
Overall, our results were counterintuitive: it is commonly accepted that acidophilic bacteria dominate low- and moderate-temperature environments, exemplified by Parys Mountain, which is exposed to the cool maritime climate. Many questions related to acidophilic Thermoplasmata still need to be addressed, e.g. the functions of predominant and “rare” community members and the reason of a very limited fraction of Thermoplasmata being cultured. Therefore, we are looking forward to sampling Parys Mountain in the near future.
1.Golyshina, O.V. et al. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order Thermoplasmatales. Int J Syst Evol Microbiol. 66(1), 332-40 (2016a).
2. Golyshina, O.V. et al. Biology of archaea from a novel family Cuniculiplasmataceae (Thermoplasmata) ubiquitous in hyperacidic environments. Sci Rep. 6, 39034 (2016b).
3. Golyshina, O.V. et al. “ARMAN” archaea depend on association with euryarchaeal host in culture and in situ. Nat Commun. 8, 60 (2017).
4. Söllinger, A. et al. Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol Ecol. 92(1), fiv149 (2016).
Follow the Topic
-
Microbiome

This journal hopes to integrate researchers with common scientific objectives across a broad cross-section of sub-disciplines within microbial ecology. It covers studies of microbiomes colonizing humans, animals, plants or the environment, both built and natural or manipulated, as in agriculture.
Related Collections
With Collections, you can get published faster and increase your visibility.
Harnessing plant microbiomes to improve performance and mechanistic understanding
This is a Cross-Journal Collection with Microbiome, Environmental Microbiome, npj Science of Plants, and npj Biofilms and Microbiomes. Please click here to see the collection page for npj Science of Plants and npj Biofilms and Microbiomes.
Modern agriculture needs to sustainably increase crop productivity while preserving ecosystem health. As soil degradation, climate variability, and diminishing input efficiency continue to threaten agricultural outputs, there is a pressing need to enhance plant performance through ecologically-sound strategies. In this context, plant-associated microbiomes represent a powerful, yet underexploited, resource to improve plant vigor, nutrient acquisition, stress resilience, and overall productivity.
The plant microbiome—comprising bacteria, fungi, and other microorganisms inhabiting the rhizosphere, endosphere, and phyllosphere—plays a fundamental role in shaping plant physiology and development. Increasing evidence demonstrates that beneficial microbes mediate key processes such as nutrient solubilization and uptake, hormonal regulation, photosynthetic efficiency, and systemic resistance to (a)biotic stresses. However, to fully harness these capabilities, a mechanistic understanding of the molecular dialogues and functional traits underpinning plant-microbe interactions is essential.
Recent advances in multi-omics technologies, synthetic biology, and high-throughput functional screening have accelerated our ability to dissect these interactions at molecular, cellular, and system levels. Yet, significant challenges remain in translating these mechanistic insights into robust microbiome-based applications for agriculture. Core knowledge gaps include identifying microbial functions that are conserved across environments and hosts, understanding the signaling networks and metabolic exchanges between partners, and predicting microbiome assembly and stability under field conditions.
This Research Topic welcomes Original Research, Reviews, Perspectives, and Meta-analyses that delve into the functional and mechanistic basis of plant-microbiome interactions. We are particularly interested in contributions that integrate molecular microbiology, systems biology, plant physiology, and computational modeling to unravel the mechanisms by which microbial communities enhance plant performance and/or mechanisms employed by plant hosts to assemble beneficial microbiomes. Studies ranging from controlled experimental systems to applied field trials are encouraged, especially those aiming to bridge the gap between fundamental understanding and translational outcomes such as microbial consortia, engineered strains, or microbiome-informed management practices.
Ultimately, this collection aims to advance our ability to rationally design and apply microbiome-based strategies by deepening our mechanistic insight into how plants select beneficial microbiomes and in turn how microbes shape plant health and productivity.
This collection is open for submissions from all authors on the condition that the manuscript falls within both the scope of the collection and the journal it is submitted to.
All submissions in this collection undergo the relevant journal’s standard peer review process. Similarly, all manuscripts authored by a Guest Editor(s) will be handled by the Editor-in-Chief of the relevant journal. As an open access publication, participating journals levy an article processing fee (Microbiome, Environmental Microbiome). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief of the journal where the article is being submitted.
Collection policies for Microbiome and Environmental Microbiome:
Please refer to this page. Please only submit to one journal, but note authors have the option to transfer to another participating journal following the editors’ recommendation.
Collection policies for npj Science of Plants and npj Biofilms and Microbiomes:
Please refer to npj's Collection policies page for full details.
Publishing Model: Open Access
Deadline: Jun 01, 2026
Microbiome and Reproductive Health
Microbiome is calling for submissions to our Collection on Microbiome and Reproductive Health.
Our understanding of the intricate relationship between the microbiome and reproductive health holds profound translational implications for fertility, pregnancy, and reproductive disorders. To truly advance this field, it is essential to move beyond descriptive and associative studies and focus on mechanistic research that uncovers the functional underpinnings of the host–microbiome interface. Such studies can reveal how microbial communities influence reproductive physiology, including hormonal regulation, immune responses, and overall reproductive health.
Recent advances have highlighted the role of specific bacterial populations in both male and female fertility, as well as their impact on pregnancy outcomes. For example, the vaginal microbiome has been linked to preterm birth, while emerging evidence suggests that gut microbiota may modulate reproductive hormone levels. These insights underscore the need for research that explores how and why these microbial influences occur.
Looking ahead, the potential for breakthroughs is immense. Mechanistic studies have the power to drive the development of microbiome-based therapies that address infertility, improve pregnancy outcomes, and reduce the risk of reproductive diseases. Incorporating microbiome analysis into reproductive health assessments could transform clinical practice and, by deepening our understanding of host–microbiome mechanisms, lay the groundwork for personalized medicine in gynecology and obstetrics.
We invite researchers to contribute to this Special Collection on Microbiome and Reproductive Health. Submissions should emphasize functional and mechanistic insights into the host–microbiome relationship. Topics of interest include, but are not limited to:
- Microbiome and infertility
- Vaginal microbiome and pregnancy outcomes
- Gut microbiota and reproductive hormones
- Microbial influences on menstrual health
- Live biotherapeutics and reproductive health interventions
- Microbiome alterations as drivers of reproductive disorders
- Environmental factors shaping the microbiome
- Intergenerational microbiome transmission
This Collection supports and amplifies research related to SDG 3, Good Health and Well-Being.
All submissions in this collection undergo the journal’s standard peer review process. As an open access publication, this journal levies an article processing fee (details here). We recognize that many key stakeholders may not have access to such resources and are committed to supporting participation in this issue wherever resources are a barrier. For more information about what support may be available, please visit OA funding and support, or email OAfundingpolicy@springernature.com or the Editor-in-Chief.
Publishing Model: Open Access
Deadline: Jun 16, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in