Unmasking the Potential of NOX Inhibitors: A Journey from Computational Design to Cancer Research
Published in Cell & Molecular Biology

Today, we're delving into the fascinating world of NADPH oxidases (NOXs), their role in cancer, and cutting-edge advancements in drug design targeting these enzymes. When controlled and harnessed strategically, reactive oxygen species (ROS) can be valuable players in multiple biological pathways. However, ROS become the antagonists of cellular health when their production spins out of control. Among the enzymes responsible for ROS production, NOXs stand out as the “professional ROS producers”. Humans possess seven NOX subtypes, each with its unique role in specific cellular processes. NOXs as orchestrators of highly localized ROS production have been implicated in a plethora of pathways such as innate immunity, redox signaling, cell proliferation, and differentiation, and their dysregulation in cancers significantly impacts ROS production, leading to redox imbalance and tumor progression.1
Why NOXs? Understanding NADPH Oxidases Mechanism and Their Significance in Cancer
Our interest in NOXs started as a structural biology interrogation mark, hoping to understand more of its regulation mechanism by mapping its three-dimensional structure. A significant breakthrough in our work happened in 2017 as our group solved the crystal structure of the cyanobacterium Cylindrospermum stagnale NOX5 (csNOX5) by examining NOX-specific catalytic domains.2 The structure nicely revealed the electron transfer chain from the intracellular space where the substrate NADPH is oxidized, to the extracellular space where the molecular oxygen is the final electron acceptor to generate ROS (Figure 1).
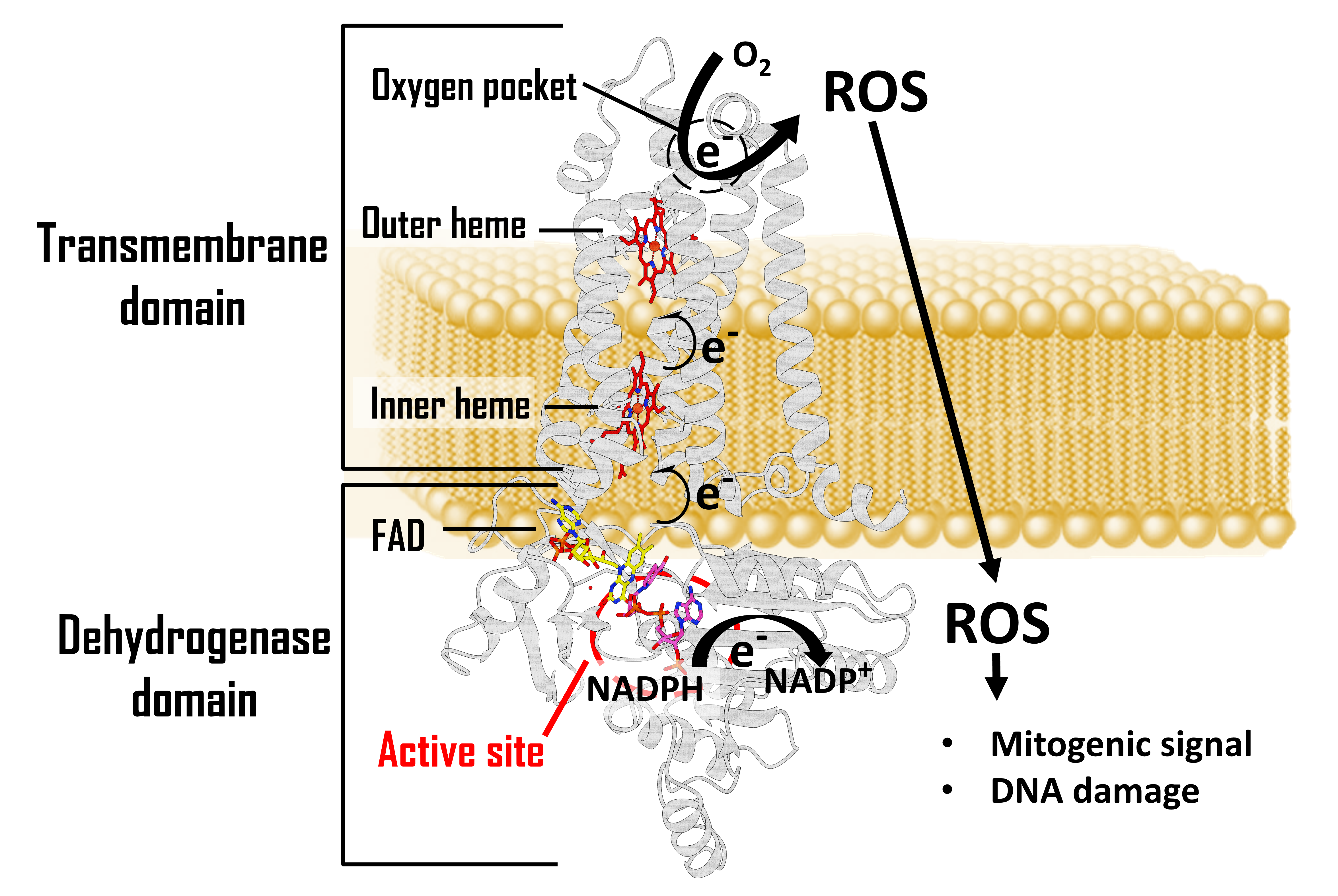
Figure 1. Domain composition of NADPH oxidases.
In the context of cancer, dysregulated NOX enzymes contribute to an abnormal increase in ROS levels, promoting tumor growth and metastasis. Therefore, targeting NOXs presents an opportunity to modulate ROS levels in cancer cells and potentially inhibit tumor progression.
In this study, we delve into a groundbreaking approach to develop effective inhibitors for the NOX family, with a focus on targeting the cytosolic dehydrogenase domain. Our journey spans four key components: in silico screening, in vitro validation, in cellulo assessment, and chemical optimization (Figure 2).
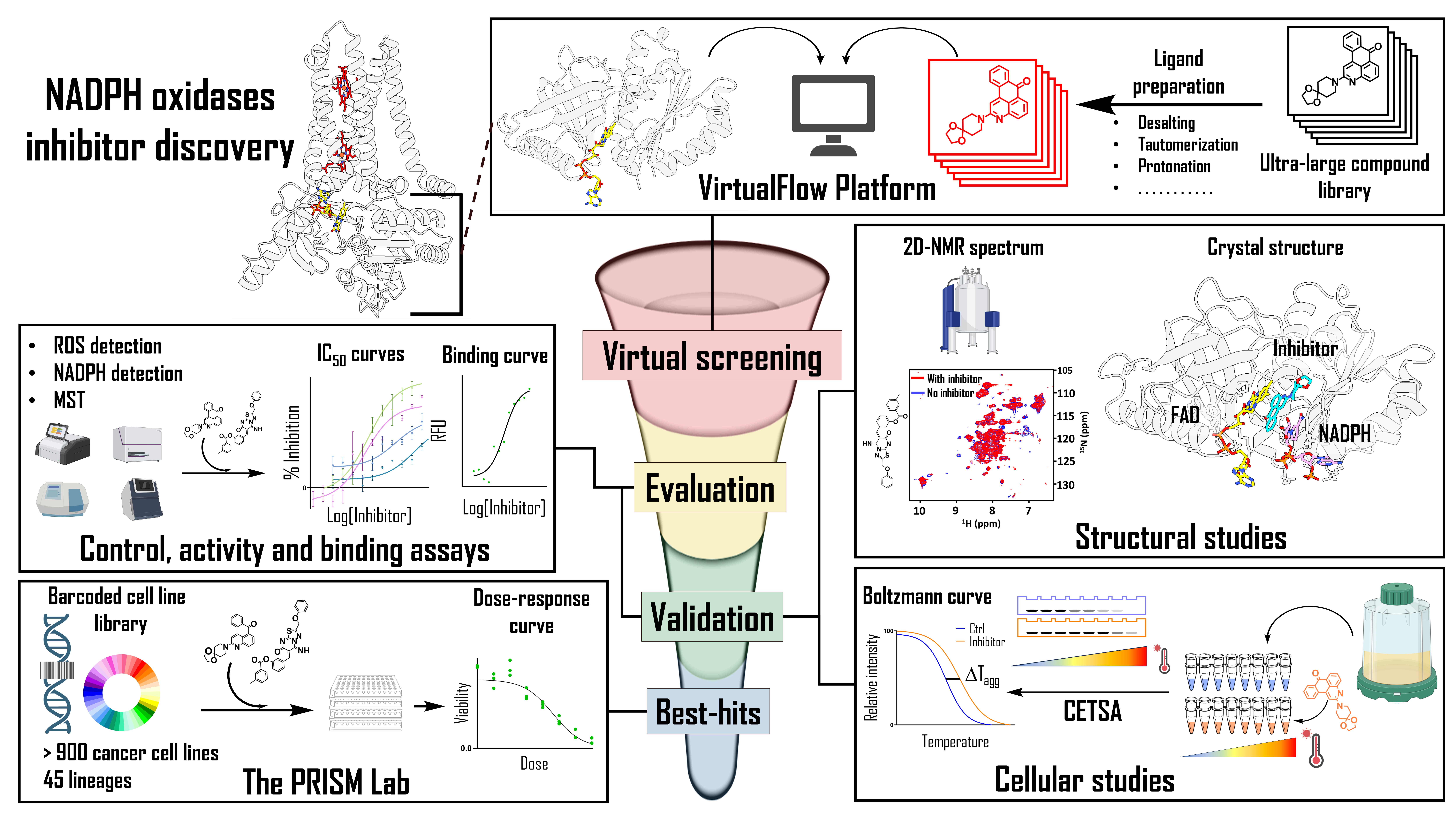
Figure 2. Experimental workflow.
In Silico Screening: A Leap Towards Targeted Drug Design
We have turned to computational methods to identify specific NOX inhibitors, particularly using the VirtualFlow in-silico screening tool.3 This technique involves computer-based simulations and virtual screening of large-sized libraries to predict and analyze the binding affinities of potential compounds to the active sites of NOX enzymes. To this end, we have successfully utilized this tool to identify potential NOX inhibitors, particularly focusing on targeting the active site of the previously mentioned bacterial NOX5 ortholog.
Validating Potential Hits: The Bridge between In Silico and Reality
Identifying potential hits through in-silico screening is just the beginning. The identified compounds must undergo rigorous validation processes to ensure their efficacy and safety. Our validation process involves in vitro and in cellulo enzymatic assays and binding assays to confirm the inhibitory effect of the compounds on NOXs. In this regard, we ventured into the realm of laboratory experimentation, establishing a robust platform for medium-throughput in vitro screening by expanding the methods already validated in the work we published in 2020.4 This allowed us to evaluate potential inhibitors across multiple human NOX homologs (NOX1, NOX2, NOX4, and NOX5). Moreover, high-resolution crystal structures have been utilized to study the binding modes of these inhibitors to the dehydrogenase domain of csNOX5, providing critical insights into their mechanism of action. Transitioning from isolated proteins to more complex systems, we also tested the inhibitors on intact cells overexpressing each human NOX.
Discovery of Bona-fide Inhibitors for Human NOXs.
Our journey yielded exciting results with the discovery of different scaffolds able to inhibit human NOXs. Different inhibitors emerged with different NOX specificity and potency, with inhibitor 3 (Figure 3) demonstrating significant efficacy against NOX4 and NOX2. Moreover, co-crystallization experiments of the csNOX5 DH with 3 and NADP+ revealed a particular mechanism of inhibition that sees the nicotinamide moiety parallel to 3, forming a three-layered stack of molecules in the active site alongside the flavin and inhibitor (Figure 3).
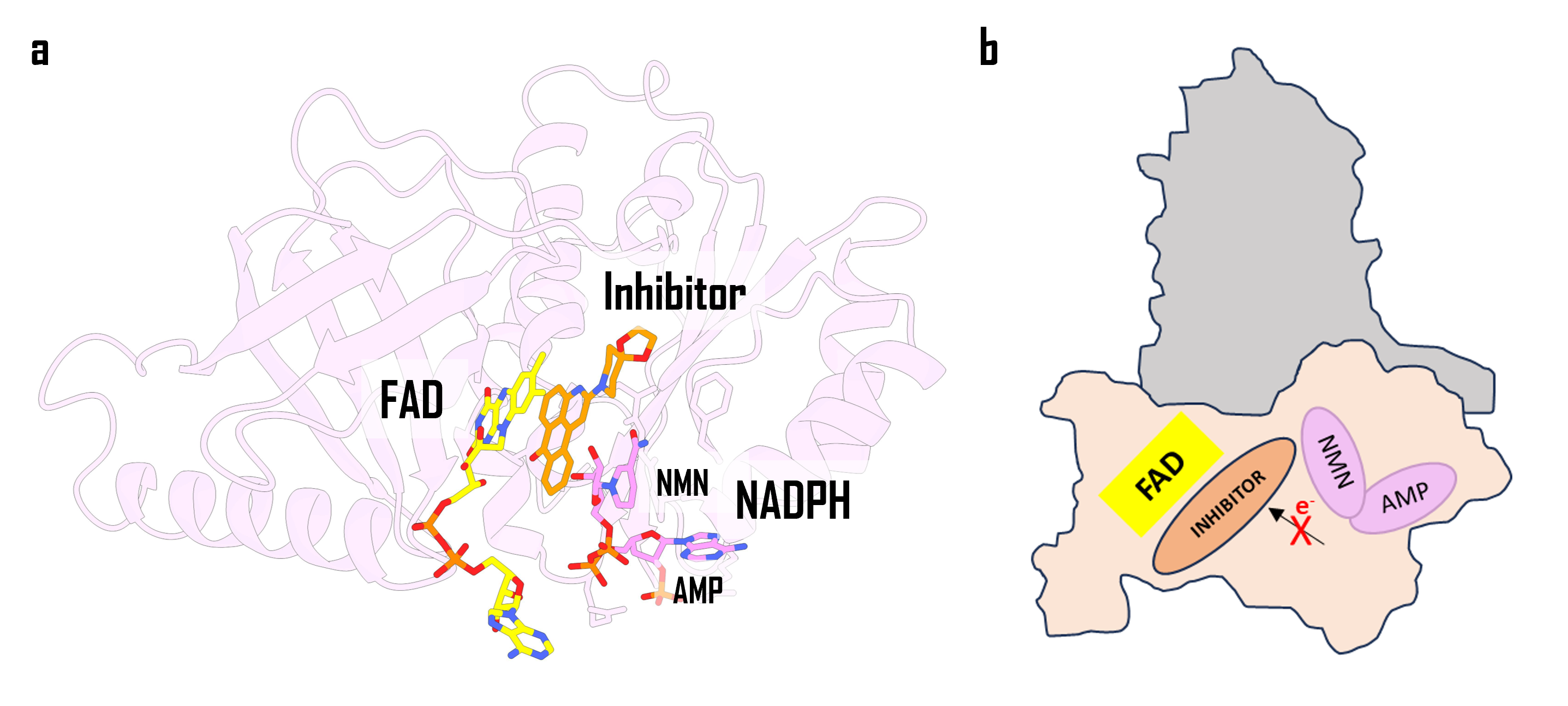
Figure 3. a) Crystal structure of the FAD-bound csNOX5 isolated dehydrogenase domain in complex with inhibitor 3 and NADP+. b) A schematic representation of the obstructed electron flow by the inhibitor. The nicotinamide portion of the substrate displays a bent position to accommodate the inhibitor.
Translating Discoveries into Clinical Potential
The real impact of the newly discovered NOX inhibitors has been realized through high-throughput screens in a panel of cancer cells using the PRISM platform (Broad Institute; https://www.theprismlab.org). The analysis showed 3 to be active in several cancer cell lines overexpressing either NOX2 or NOX4. These results further moved us beyond the analysis of correlative associations between ROS and human cancers. ROS are indeed known to fuel proliferative signaling and are required for cell transformation and tumorigenesis by oncogenes such as oncogenic Ras5. Therefore, we sought to determine whether NOX inhibition would complement the activity of KRAS modulators. We combined NOX inhibitor 3 with KRASG12D mutant-specific modulators in colorectal cancer cell lines. Remarkably, even at low concentrations, the combination of KRASG12D modulators and 3 significantly enhanced therapeutic efficacy. By exploring the synergistic effects of these inhibitors in combination with KRAS modulators, we are paving the way for a potential therapeutic strategy in cancer treatment.
A Glimpse into the Future: Inhibitor-Based Methods for ROS Control
The discovery of bona-fide NOX inhibitors opens a realm of possibilities for precision medicine, allowing us to modulate or suppress NOX signaling without interfering with non-specific ROS scavenging or antioxidant effects. Our work also hints at the potential for synergizing NOX inhibitors with drugs targeting specific oncogenes or pathways, benefiting the current cancer treatments in place. In conclusion, the convergence of computational techniques, biochemical validation, and cellular assays is propelling the field of NOX drug design toward more targeted and effective cancer therapeutics.
1. Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. 21, 363-383 (2020).
2. Magnani, F. et al. Crystal structures and atomic model of NADPH oxidase. PNAS 114, 6764-6769 (2017).
3. Gorgulla, C. et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 580, 663-668 (2020).
4. Reis, J. et al. A closer look into NADPH oxidase inhibitors: Validation and insight into their mechanism of action. Redox Biol. 32, 101466 (2020).
5. Lim, J. K. M. & Leprivier, G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 10, 955 (2019).
Follow the Topic
-
Nature Chemical Biology

An international monthly journal that provides a high-visibility forum for the chemical biology community, combining the scientific ideas and approaches of chemistry, biology and allied disciplines to understand and manipulate biological systems with molecular precision.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in