Unveiling the Dance of Atoms: The Intricate Dynamics of Gold Nanoclusters in Catalysis

In the ever-growing quest for greener energy solutions, hydrogen has emerged as a promising candidate, boasting high energy content and potential for clean fuel production. Among the various methods, the water-gas shift (WGS) reaction stands out. However, achieving efficient WGS catalysts requires a deep understanding of catalyst structures at the atomic level.
Ligand-protected metal nanoclusters have recently captured the spotlight, offering atomic precision, well-defined structures, and unique molecular-like properties. In our recent paper, we studied the dynamic behavior of platinum and copper dopants within gold nanoclusters supported on ceria catalysts, with a focus on their role in the WGS reaction.
We synthesized and characterized four types of nanoclusters: monometallic Au, bimetallic CuAu, PtAu, and trimetallic CuPtAu nanoclusters (Figure 1), which were then supported on ceria and tested as catalysts in the WGS reaction.
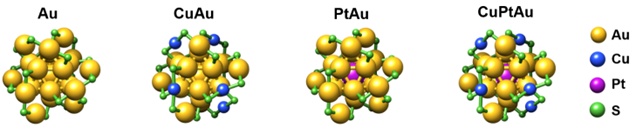
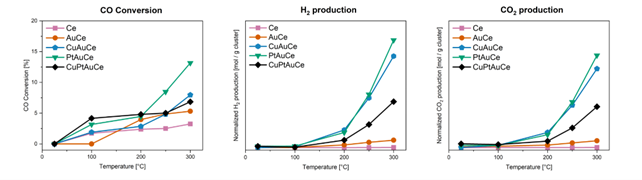
The first suprise came with the results of the catalytic activity. Bimetallic PtAu (and CuAu) clusters outperformed their trimetallic counterparts, showing increased activity and selectivity. The dance of these atoms continued through multiple catalytic runs, with PtAu clusters demonstrating remarkable stability compared to their trimetallic counterparts.
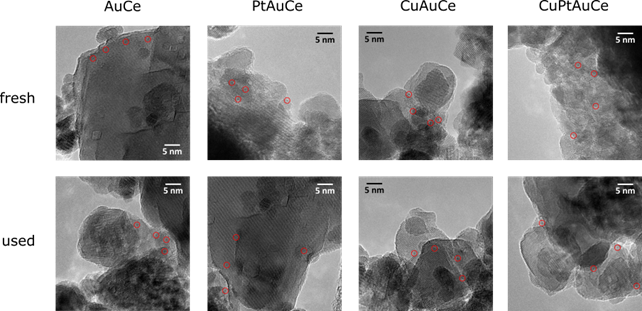
Furtermore, high-resolution transmission electron microscopy (HRTEM) studies provided a glimpse into the atomic world of these nanoclusters. The subnanometric size and excellent dispersion of Au clusters on ceria support remained unchanged even after the WGS reaction, showcasing the stability of these catalysts.
Using operando X-Ray absorption fine sturcture spectroscopy (XAFS), we were able visualize the movement of the dopants during the catalytic process. Now picture this: as we subjected our catalysts to pretreatment and the WGS reaction, copper decided to take a journey. It migrated, forming clusters on the ceria support. On the other hand, platinum showed off its moves by creating single-atom active sites on the gold cluster surface. The result? Enhanced catalytic performance.
While the XAFS analysis was anything but trivial, we were able to unravel this mysterious ballet of atoms and display it in the following Figures.
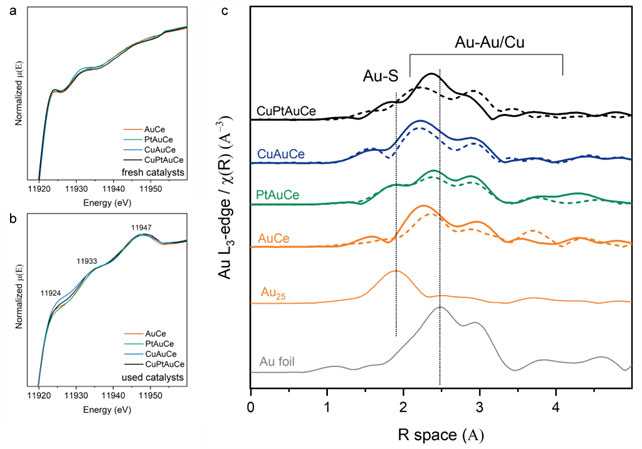
Espacially the extended X-Ray absorption fine structure spectroscopy (EXAFS) seen in Figure 4c already hints at some unexpected changes. The Au-Au coordination number (CN) increases after the WGS rreaction from 6 to 7-8 for the bimetallic and even to 10 for the trimetallic clusters. But we cannot yet say anything for certain.
This is where the Cu-K edge analysis comes into play. Looking at the X-Ray absorption near edge structure spectroscopy (XANES) study in Figure 5, the dance of the copper atoms starts to gain more clarity. The strong interaction of the dopants with the ceria surface can be seen.
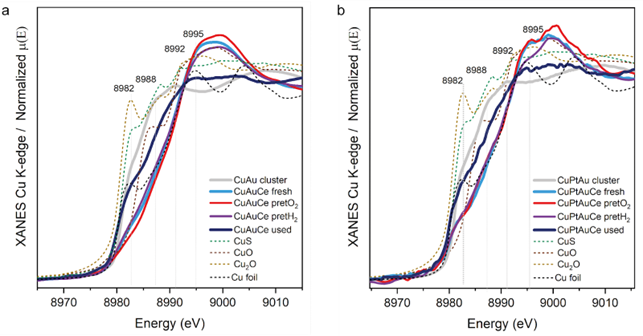
To fully understand the performance, the EXAFS of the Cu-K edge is needed. Here, an increase in the CN of Cu-Cu is visible, which further coroborates the aforementioned affirmations. But to see this, as well as an equally interesting operando DRIFTS study of these nanoclusters, we invite you to read the paper linked at the top of this post.
To conclude, finding new energy sources is of great importance for the future of the world we live in and hydrogen is a great candidate. The WGS shift reaction is a great way to produce it, but a deep understanding of the catalyst is needed to fully maximize its efficiency. Understanding the intricate dynamics of the dopants in these nanoclusters, thus unveiling the dance of these atoms helps us on our way to design an increasingly effective catalyst.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Your space to connect: The Polarised light Hub
A new Communities’ space to connect, collaborate, and explore research on Light-Matter Interaction, Optics and Photonics, Quantum Imaging and Sensing, Microscopy, and Spectroscopy!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in