Unveiling the mechanism of SIDT1 in cellular uptake of dsRNA
Published in Cell & Molecular Biology
Explore the Research
Human SIDT1 mediates dsRNA uptake via its phospholipase activity - Cell Research
Cell Research - Human SIDT1 mediates dsRNA uptake via its phospholipase activity
RNA interference (RNAi) refers to the gene silencing triggered by dsRNA, which not only plays essential roles in numerous biological processes including cell development, antiviral immunity and stress responses, but also has been used as a powerful genetic tool to investigate gene function, to control pest insect and to treat diverse diseases in clinic. Andrew Z. Fire and Craig C. Mello were awarded the Nobel Prize in Physiology or Medicine of 2006 for their contributions in the discovery of RNAi.
RNAi could spread throughout the organism and its progeny, which is also termed systemic RNAi1,2. Hunter and colleagues identified that a ubiquitously expressed putative transmembrane protein SID-1 (systemic RNAi defective-1) in Caenorhabditis elegans is required for systemic RNAi3, probably via acting as a channel to passively transport extracellular dsRNA into cells4,5. SIDT1 (SID-1 transmembrane family member 1) is a SID-1 homolog in human. Previous reports showed that it could facilitate the cellular uptake of both short interfering RNAs (siRNAs) and microRNAs (miRNAs)6-8. Given a sequence identity of ~24%, SIDT1 might function in a way similar to nematode SID-1. However, the molecular mechanism of either SID-1-mediated RNAi or SIDT1-facilitated dsRNA uptake remains unknown.
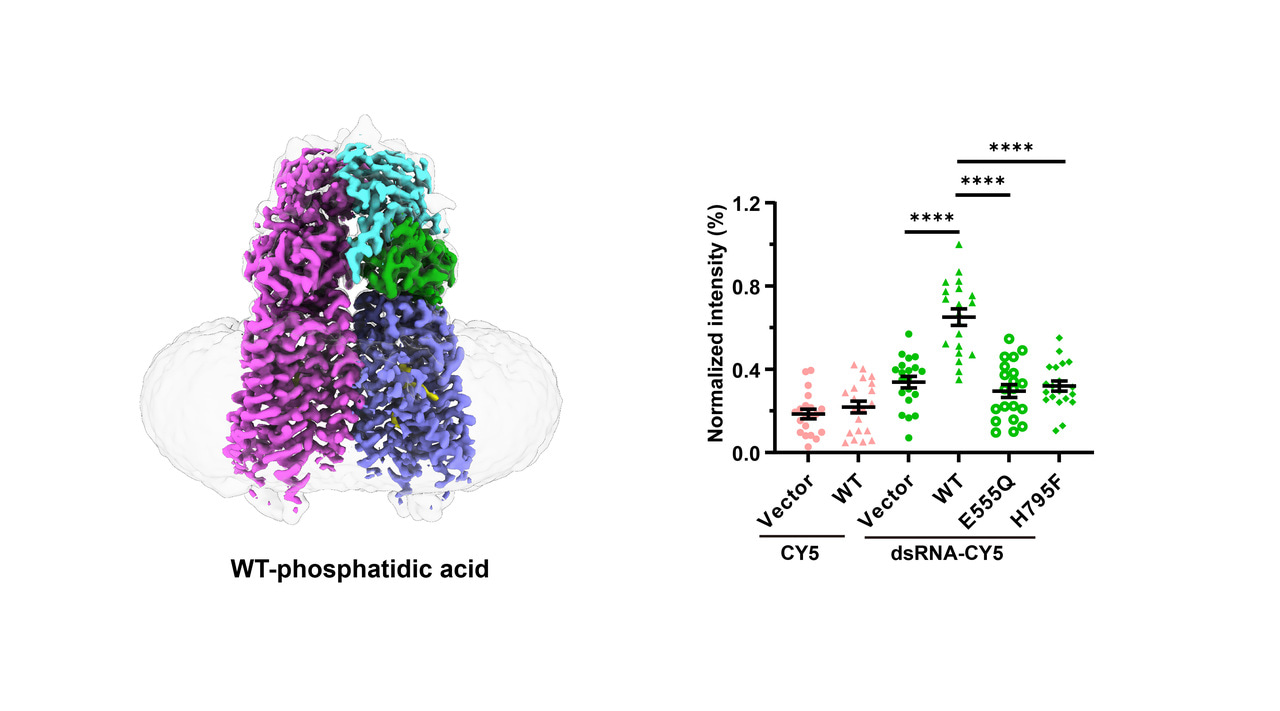
In this study, we solved two cryo-EM structures of human SIDT1: the wild-type structure complexed with phosphatidic acid (PA) at a resolution of 2.9 Å, and the E555Q mutant at 2.4 Å. These structures together with a series of in vitro experiments not only identify SIDT1 as a novel transmembrane phospholipase, but also elucidate a unique phospholipase-dependent mechanism that enable SIDT1 to mediate the uptake of dsRNA.
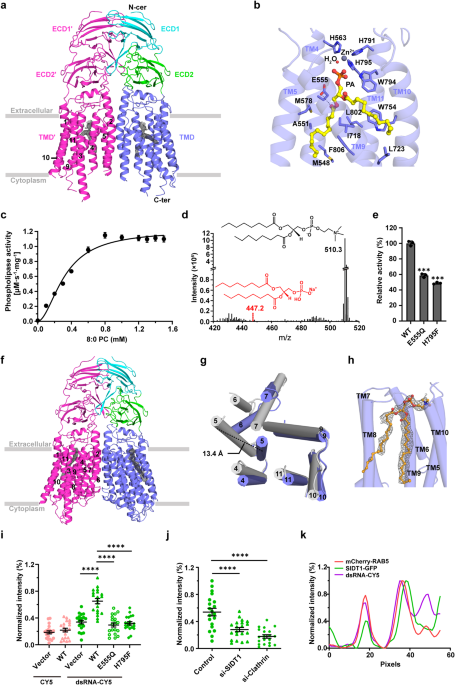
Human SIDT1 adopts a dimeric structure, each subunit of which contains two extracellular domains (termed ECD1 and ECD2) and a transmembrane domain (TMD) of 11 transmembrane helices. Structural superposition proved that SIDT1 is indeed a member of the superfamily CREST of putative transmembrane hydrolases, but functions as a homodimer and adopts a reversed topology. Moreover, a PA molecule, a Zn2+ ion and a water molecule were accommodated in the pocket of each TMD, which further enable us to identify that SIDT1 is a Zn2+-dependent phospholipase D (PLD), combined with enzymatic and mass spectrometry assays. Afterwards, structure of the E555Q mutant suggests a lateral access of the substrate. Furthermore, a series of fluorescence assays indicate that SIDT1 mediates the uptake of either dsRNA, miRNA or dsDNA, via the phospholipase activity.
These findings not only advance our understanding on both CREST and PLD superfamilies, but also enable us to identify a clade of transmembrane phospholipase and an uptake pathway via trimming the membrane phospholipids. Our study on SIDT1 will help elucidate the molecular mechanism of nucleic acid transport and RNAi mediated by SID-1. Moreover, as a transmembrane phospholipase, SIDT1 might also be applied to drug delivery, especially in the field of RNA therapeutics.

Fig.1 Structures of human SIDT1 and its phospholipase activity dependent dsRNA uptake. a Cartoon representation of wild-type SIDT1 complexed with phosphatidic acid (PA). b The PA-binding pocket. c The phospholipase activity of SIDT1 upon addition of the 8:0 phosphatidylcholine (PC) at various concentrations. d Mass spectrum of SIDT1 plus the 8:0 PC sample. e Relative phospholipase activities of SIDT1 and mutants towards the 8:0 PC. f Cartoon representation of SIDT1E555Q. g Superposition of TMs with conformational changes of the wild-type SIDT1 (gray) against SIDT1E555Q (blue) in a view from the cytoplasm. h The hydrophobic POPC-binding pocket. i-j Statistical analyses against the uptake of CY5 and dsRNA-CY5 in HEK293T cells transfected with i GFP vector, wild-type SIDT1 (WT) or mutant (E555Q/H795F) plasmid, and j SIDT1 or Clathrin siRNA. l Colocalization of SIDT1 with the early endosome (RAB5 as marker) upon dsRNA-CY5 treatment.
References
1 Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806-811 (1998).
2 Tabara, H., Grishok, A. & Mello, C. C. RNAi in C. elegans: Soaking in the Genome Sequence. Science 282, 430-431 (1998).
3 Winston, W. M., Molodowitch, C. & Hunter, C. P. Systemic RNAi in C. elegans Requires the Putative Transmembrane Protein SID-1. Science 295, 2456-2459 (2002).
4 Feinberg, E. H. & Hunter, C. P. Transport of dsRNA into Cells by the Transmembrane Protein SID-1. Science 301, 1545-1547 (2003).
5 Shih, J. D. & Hunter, C. P. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA 17, 1057-1065 (2011).
6 Duxbury, M. S., Ashley, S. W. & Whang, E. E. RNA interference: A mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem. Biophys. Res. Commun. 331, 459-463 (2005).
7 Wolfrum, C. et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 25, 1149-1157 (2007).
8 Chen, Q. et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 31, 247-258 (2021).
Follow the Topic
-
Cell Research

This journal publishes original research results that are of unusual significance or broad conceptual or technical advances in all areas of life sciences, as long as the study is closely related to molecular and cell biology.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in