USP33 facilitates the ovarian cancer progression via deubiquitinating and stabilizing CBX2
Published in Biomedical Research
Ovarian cancer is one of the leading causes of gynecological malignancies worldwide, with approximately 75% of patients presenting with advanced disease and extensive metastasis, resulting in a low 5-year survival rate. This underscores the urgent need to identify novel therapeutic targets for the treatment of patients with metastatic ovarian cancer. The role of USP33 in carcinogenesis is complex and dualistic; however, its role and underlying mechanisms in ovarian cancer progression have not yet been thoroughly investigated. Given that USP33 functions as a deubiquitinating enzyme, we aim to explore its role and mechanisms in ovarian cancer from the perspective of post-translational protein modification.
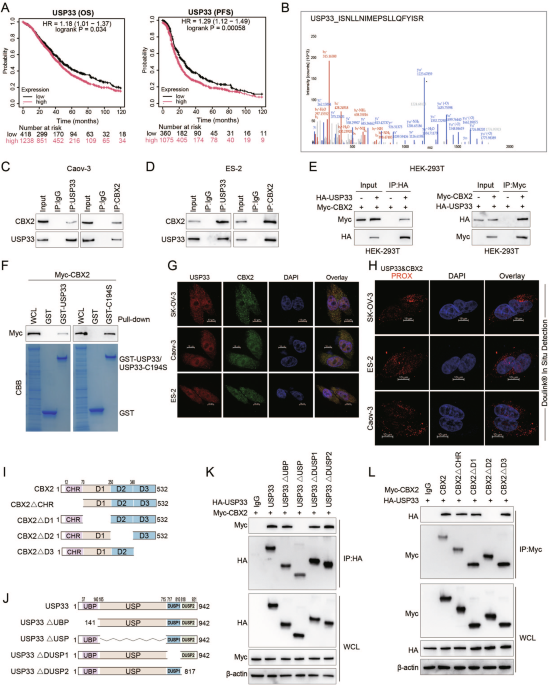
First, we employed proteomics and ubiquitinomics approaches to screen for novel substrate proteins directly regulated by USP33. Through a series of experiments, we confirmed the interaction between USP33 and CBX2. We also observed that the USP domain of USP33 and the CBX2-D2 domain are essential for their interaction. Our findings further elucidate that USP33 facilitates the deubiquitination and subsequent stabilization of CBX2. Additionally, we identified lysine 199 (K199) of CBX2 as a highly conserved acetylation site across multiple species, as indicated by data from the PhosphoSitePlus® PTM database. We then investigated whether CBX2 could be acetylated at K199 and whether this acetylation affects USP33-mediated CBX2 ubiquitination. We demonstrated that the acetylation of CBX2 at lysine 199, catalyzed by the lysine acetyltransferase GCN5, enhances its association with USP33, thereby promoting further deubiquitination and stabilization.
Next, we explored the functional role of USP33 in ovarian cancer. Through in vitro cell experiments and mouse models, we demonstrated that USP33 significantly enhances ovarian cancer proliferation and metastasis in a CBX2-dependent manner. Furthermore, we employed immunohistochemistry to assess the expression of USP33 and CBX2 in clinical tissue samples and analyzed their correlation with prognosis. Our findings revealed a direct positive correlation between the expression levels of USP33 and CBX2 proteins in human specimens, with elevated levels being associated with reduced survival rates in ovarian cancer patients.
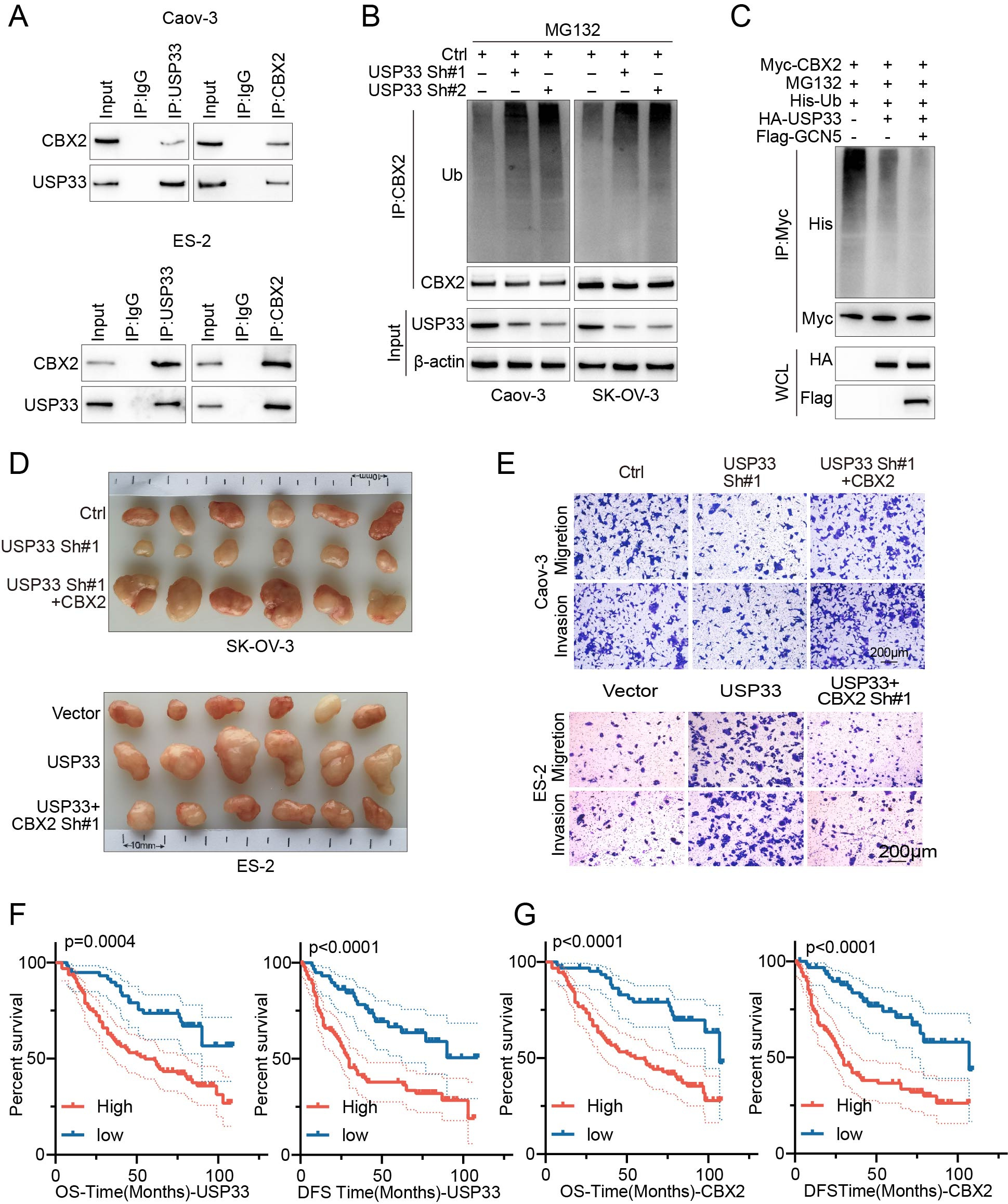
In summary, our research elucidates the pivotal role of USP33, an oncogenic factor, in ovarian cancer by facilitating the deubiquitination and stabilization of CBX2. Noteworthy, we demonstrate that the acetylation of CBX2 at the K199 site, catalyzed by GCN5, enhances its interaction with USP33, resulting in augmented deubiquitination and stabilization of CBX2. These findings underscore that USP33 is a promising drug target, and inhibiting USP33 activity and mitigating CBX2-dependent tumorigenicity potentially offer a promising avenue for ovarian cancer therapy.

This study’s innovation lies in its comprehensive elucidation of the molecular mechanisms by which USP33 promotes tumor progression through post-translational modifications, thereby providing a significant theoretical basis for future translational applications. However, several avenues for further research remain. Firstly, PhosphoSitePlus® PTM data have identified phosphorylation as another prevalent post-translational modification for CBX2. Substantial evidence suggests that the interplay between phosphorylation and ubiquitination often augments protein stability and interactions. Whether the synergy between these modifications influences CBX2 stabilization in ovarian cancer warrants further investigation. Secondly, although the oncogenic role of USP33 in ovarian cancer has been elucidated, the precise mechanisms underlying its overexpression remain unclear, necessitating further inquiry. Thirdly, it is crucial to explore the downstream targets or pathways activated as a result of CBX2 stabilization. Finally, the development of a safe and effective USP33 inhibitor is of paramount importance for assessing its potential as a therapeutic target.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in