Vaccines & HIV: Updated Recommendations
Published in General & Internal Medicine, Immunology, and Public Health

Explore the Research
Update on Vaccination Recommendations for Adults with HIV
This post was written in honor of HIV Vaccine Awareness Day on May 18, which recognizes the ongoing efforts to develop a safe and effective HIV vaccine—and highlights the broader importance of immunization in people living with HIV.
We are infectious diseases physicians at Weill Cornell Medicine, where we also provide primary care for people living with HIV. As both clinicians and researchers, we are deeply engaged in understanding how immunization strategies can be optimized for this population. This perspective shaped our recent review, published in Current HIV/AIDS Reports, which explores how and why vaccination recommendations for people with HIV (PWH) differ from those for the general population.
Importantly, even with effective antiretroviral therapy and undetectable viral loads, PWH experience persistent immune differences — subtle but meaningful changes that influence how well vaccines work and how they should be delivered. This work was driven by a central question: How do we protect people whose immune systems function well enough to avoid most infections, but not well enough to respond optimally to all vaccines?
What We Did
In this review, we summarized evolving vaccine recommendations for adults with HIV, drawing from national guidelines and recent trials. We focused on how persistent immune dysfunction in people with HIV - such as reduced numbers of memory B cells (antibody-producing cells), chronic inflammation, and incomplete CD4 recovery - diminishes vaccine effectiveness and necessitates different timing, dosing, and monitoring strategies compared to the general population.
Some of the most notable key differences from recommendations for the general population include:
-
Pneumococcus: All people with HIV should receive pneumococcal vaccination, regardless of age or CD4 count—a broader recommendation than for the general population. PCV21, the newest vaccine option, provides the widest coverage to date, including strains responsible for over 80% of invasive disease in high-risk adults.
-
Mpox: Vaccination is recommended for PWH with advanced or untreated HIV, as well as for anyone with certain risk factors like recent STIs, multiple sexual partners, or exposure through sexual networks or events. The vaccine is safe and effective across CD4 counts.
-
RSV: A newly approved vaccine for RSV is now recommended for routine use in adults aged 75 and older. For PWH aged 60-74, it may also be considered if they have other risk factors - like advanced or untreated HIV, chronic heart or lung disease, diabetes, or obesity.
-
Hepatitis B: The CpG-adjuvanted vaccine (Heplisav-B) is now preferred to other hepatitis B vaccines due to its stronger and more reliable immune response in PWH. Both hepatitis B and hepatitis A vaccination are recommended for all PWH.
- VZV (Shingles) and Meningococcus: The recombinant zoster (shingles) and meningococcal ACWY vaccines (MenACWY) are recommended for all adults with HIV. This differs from general population guidelines, where shingles vaccination is typically recommended starting at age 50 and MenACWY is advised only for those with specific risk factors.
We highlighted these recommendations alongside longstanding guidance for other vaccines, including HPV, influenza, Covid-19, and MMR, and TDaP. For each vaccine, we examined both disease risk and data on immune response in people with HIV, and provided updated guidance based on recent ACIP and CDC recommendations.
Our work also explored nuances like when to measure serologic responses, the benefits of newer adjuvants (e.g., AS01, CpG 1018), the potential of some vaccines to provide broader protection (e.g., one type of meningitis vaccine may provide some protection against gonorrhea), and the importance for monitoring for rare but serious adverse events, such as Guillain-Barré Syndrome following RSV vaccination. While much of this may seem technical, the bottom line is straightforward: optimizing vaccine strategies are essential to protect a population that experiences early immune aging and elevated infection risk.
Summary of Vaccine Considerations for Adult People with HIV
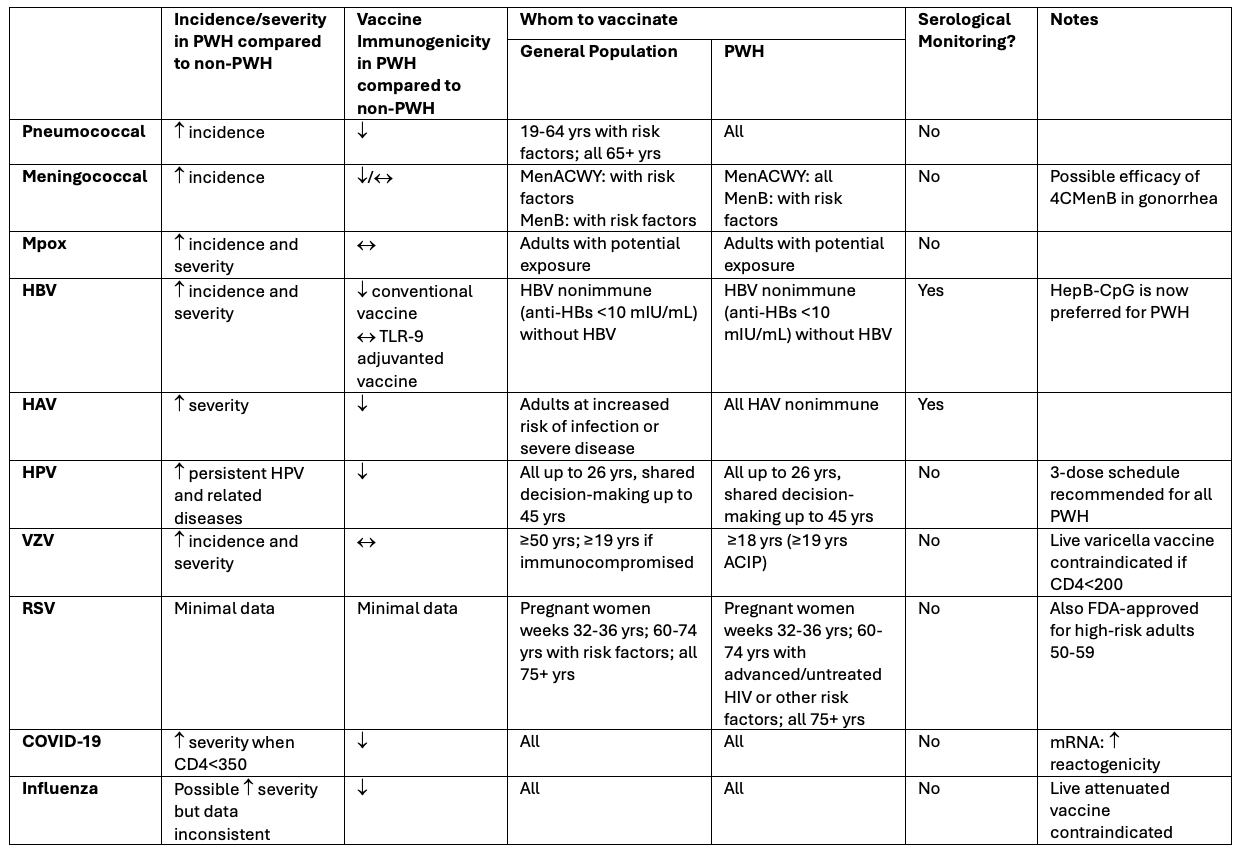
For more information see individual vaccination recommendations at www.cdc.gov/vaccines/index.html or DHHS Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV available at https://clinicalinfo.hiv.gov/
Why It Matters
Many people with HIV face obstacles when it comes to getting vaccinated. These may include not having a regular healthcare provider, unclear information about which vaccines they need, or trouble getting to appointments. Vaccine hesitancy is also a growing concern—fueled by mistrust in the healthcare system, prior negative experiences, and the spread of misinformation. And even when vaccines are recommended, they’re not always offered. These issues, combined with more complex vaccine schedules, make it harder for PWH to get the protection they need.
This paper is a reminder that undetectable does not mean invincible. Even with early ART and high CD4 counts, PWH often experience faster immune aging (immunosenescence), chronic inflammation, and reduced vaccine responses compared to the general population. These immune alterations may not be visible to the eye or detectable with routine labs, but they have real clinical implications.
This is especially true as new vaccines are introduced. Without specific data on PWH, assumptions based on the general population may lead to under-vaccination or missed opportunities. Our review underscores the need for more data on vaccine responses in PWH and supports greater inclusion of this population in vaccine trials. It also highlights the critical role of clinicians in staying engaged—offering clear guidance, ensuring access, and adapting vaccination plans as new evidence emerges.
Ultimately, vaccines are one of our most powerful tools to prevent disease in this population. But to use them effectively, they need to be delivered at the right time—and backed by strong, confident recommendations from providers who understand the unique needs of people with HIV.
Clinical Guidelines and References
- National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America: Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. . https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection (The guidelines include a section on Immunizations for Preventable Diseases in Adults and Adolescents with HIV that was updated April 23, 2025.)
- University of Washington National HIV Curriculum on Immunizations: https://www.hiv.uw.edu/go/basic-primary-care/immunizations/core-concept/all
- CDC Interactive Guideline Tool on Pneumococcal Vaccination: https://www2a.cdc.gov/vaccines/m/pneumo/pneumo.html
- Centers for Disease Control and Prevention. Vaccines and Immunizations: Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (Frequently updated source for COVID vaccination recommendations.)
Follow the Topic
-
Current HIV/AIDS Reports

Current HIV/AIDS Reports provides in-depth review articles contributed by international experts on the most significant developments in the field.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Topical Collection on HIV Pathogenesis and Treatment
Publishing Model: Hybrid
Deadline: Ongoing
Topical Collection on The Science of Prevention
Publishing Model: Hybrid
Deadline: Ongoing



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in