Volumetric imaging with uniform 3D resolution across scales
Published in Bioengineering & Biotechnology
Light-sheet fluorescence microscopy (LSFM) has found applications for imaging subcellular dynamics, embryogenesis, organoids, as well as imaging whole organs and model organisms using tissue clearing. However, the resolution in the third dimension is tied to its field of view and is typically worse than the lateral one. Axially swept light-sheet microscopy (ASLM) overcomes the trade-off between field of view and resolution in the third dimension, providing 3D imaging with uniform resolution over large volumes.
The essence of LSFM is to shape the excitation light into a sheet, which is used to optically section the sample. By only illuminating a thin slice, which coincides with the focal plane of a detection microscope, the overall irradiation of the sample is minimized, and a sharp image devoid of out-of-focus blur can be rapidly acquired with sensitive scientific cameras.
Ideally, this light-sheet should be as thin as possible, as the sectioning thickness governs the resolution in the third dimension. However, light does not like to be confined, and the tighter one tries to focus it, the stronger it diverges. Thus, a tightly focused laser beam can only approximate over a short distance a uniform sheet (over the so-called beam waist) before it fans out again. Consequently, if one wants to observe a large region using LSFM, one must contend with a thick light-sheet.
In ASLM, we do not overcome the physics that govern the formation of the light-sheet. Instead, for a desired thickness, we accept that the beam waist is finite, and may only cover a portion of the desired field of view (FOV). To fill the full FOV, we scan the light-sheet forward (in the direction the laser light propagates) and simultaneously read out the fluorescence information from the beam waist as it sweeps over the camera sensor (Figure). This is done with a so-called rolling shutter mode of the camera, where the camera frame is read out line by line instead of transferring the entire frame at once. This mode allows us to capture only the information stemming from the beam waist as it is being scanned, but not the blur before and after the waist where the laser sheet is fanning out. As a result, ASLM can decouple FOV and 3D resolution in light-sheet microscopy, and provide imaging over large volumes with isotropic resolution, i.e., the resolution is the same in any spatial dimension.
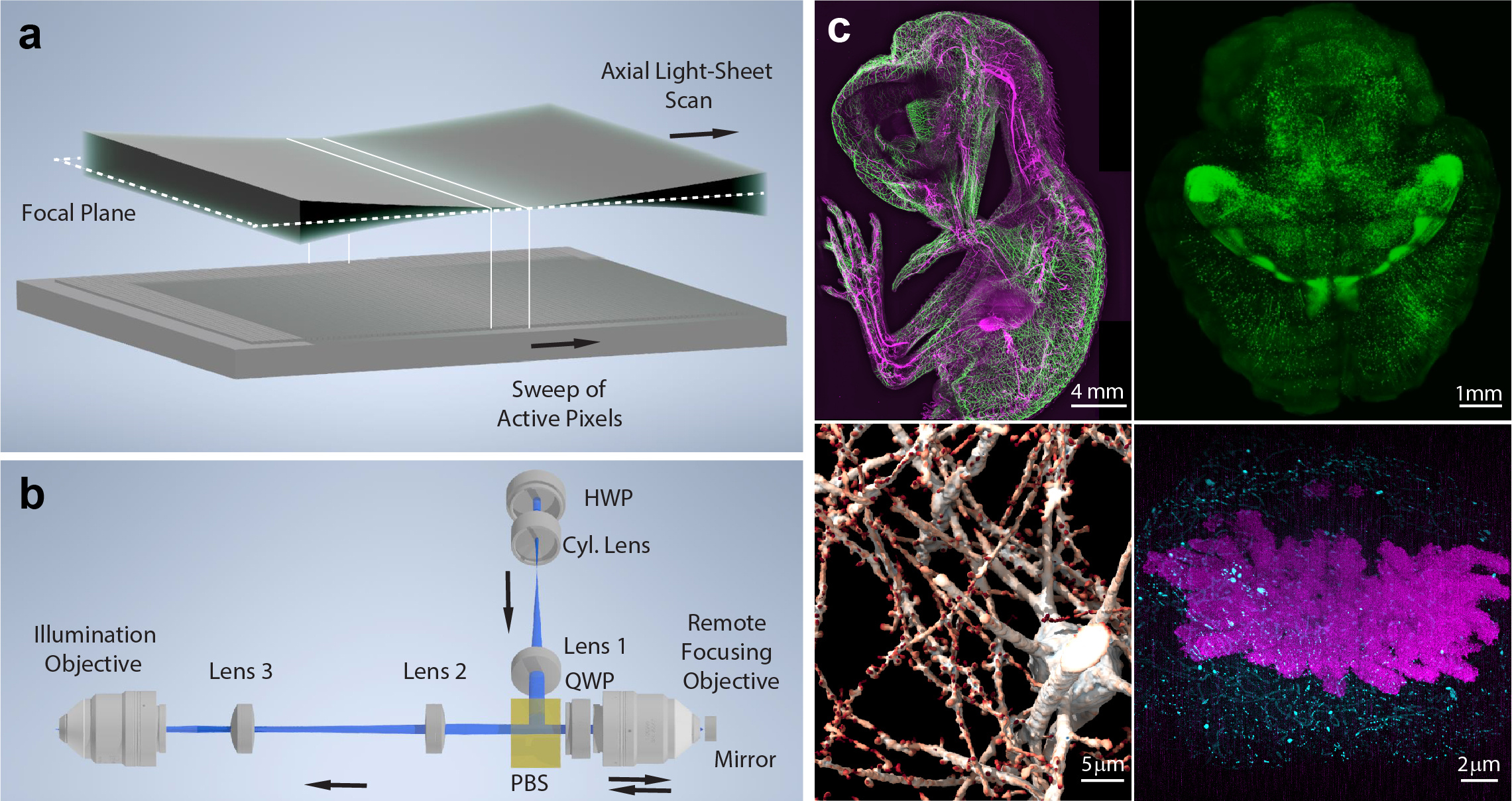
Axially swept light-sheet microscopy (ASLM)
a) Working principle of ASLM: A thin light-sheet, which coincides with the focal plane of the detection system, is scanned in its propagation direction. On the camera (schematically shown below), a rolling shutter is synchronized with the beam waist of the light-sheet as it is being scanned. b) Schematic drawing of the remote focusing system employed to scan the light-sheet in its propagation direction. A cylindrical lens shapes a laser beam into a light-sheet, which is imaged via a remote focusing objective onto a movable mirror. The image of this light-sheet is mapped via two lenses and the illumination objective into the sample plane. As the mirror is moved, the image of the light-sheet moves as well. A half and a quarter wave plate (HWP and QWP) as well as a polarizing beam splitter are used to couple the light into the remote objective and then transfer it to the illumination objective. c) Examples of ASLM imaging across scales. Clockwise starting top left: Whole animal imaging of neurofilaments in a chicken embryo with ~5 micron resolution; Whole tissue imaging of a mouse brain with ~700 nm resolution; Chromosomes imaged with ~20 nm resolution in an expanded mitotic HeLa cell; Synaptic-level imaging in a CLARITY cleared mouse brain.
To perform rapid scanning of the light-sheet in its propagation direction, which essentially means continuously re-focusing it somewhere else, we employ a method called remote focusing. Here, we focus the laser light through a remote objective onto a mirror, which is imaged over additional optics into the sample plane. When the mirror is moved, the laser focus is shifted, and its shifted image is mapped into the sample plane. If certain optical conditions are met, this refocusing of the laser beam happens without any distortions or loss of resolution (Figure).
In practice, ASLM allows us to synthesize thin light-sheets over large field of views. However, as with everything, this comes at a price. The higher the aspect ratio of the resulting sheet (i.e., scanning a thin sheet over a large field of view), the shorter the time becomes when the pixels of the camera integrate a fluorescent signal from the sample. Thus, the more one pushes ASLM, the weaker the signal becomes. Furthermore, as the light-sheet is scanned, every part of it excites fluorescence, both the beam waist that we record, but also its blurrier, fanned out parts, which we do not record. As such, more unwanted fluorescence is excited, which goes unused.
As such, the scan range in ASLM must be judiciously adjusted in life cell imaging experiments. In doing so, our lab has imaged in 3D the morphology and signaling of cancer cells, and aided by the isotropic resolution, this data has been analyzed by computer vision algorithms. With the advent of tissue clearing and expansion, large tissues and entire organisms are now within reach of optical microscopy. To efficiently image mm to cm sized volumes of cleared tissue, LSFM plays a crucial role. Here we found that ASLM is well suited to quickly image large volumes while providing isotropic resolution. In a tissue, there is no preferred orientation of cellular features (unlike cells cultured on a coverslip). Hence, to enable unbiased analysis of the data, isotropic resolution is paramount. We have developed a wide range of ASLM systems, ranging from mesoscopic imaging of cm sized cleared organisms down to high-resolution variants with subcellular resolution (Figure).
In summary, ASLM is a technique that offers isotropic 3D resolution and high contrast over large volumes. It can be applied for imaging of subcellular features in 3D live cell imaging to entire organs and model organisms using tissue clearing. Through this protocol, we hope to enable the widespread application of this technology. A "tweetorial" about the key parts of the protocol can be found here: https://twitter.com/RetoPaul/status/1547250555118133250
By Reto Fiolka, UT Southwestern Medical Center
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in