What can horned beetles tell us about the mechanisms of plasticity and their evolution?
Published in Ecology & Evolution

How do organisms interact with their environments? Research on phenotypic plasticity focuses on how organisms with the same genotype can adjust their phenotypes in response to different environmental conditions. Plasticity can take many forms and can occur at any stage during development, but those changes that occur early in development are commonly irreversible and it is those changes that fall under the rubric of developmental plasticity.
An extreme case of developmental plasticity are polyphenisms, where instead of showing a gradual response to a particular environmental factor, individuals develop into distinctly different, alternative phenotypes. One of the most well-known examples of polyphenism are castes in social insects (bees, ants, etc.), in which nutritional conditions determine whether females with the same genotype develop into workers or queens, with no intermediates.
Yet, what are the mechanisms that specify development of one or the other phenotype? And, how does plasticity itself evolve? Are the genetic mechanisms underlying plasticity responsible for the different degrees of plasticity seen across species?

Three species of horned beetles used: Digitonthophagus gazella, Onthophagus taurus and Onthophagus sagittarius (from left to right).
I have been tackling these questions for the past several years. Our recently published article in Nature Ecology and Evolution was the last chapter of my PhD dissertation, and tried to answer some of these questions from a comparative transcriptomic approach. My advisor, Armin Moczek, and I decided to collaborate with Eduardo Zattara, a former postdoc in the lab who has ample experience in analyzing transcriptomes. Despite having returned to Argentina, Eduardo was the perfect mentor to remotely guide me through all the bioinformatic tools needed for this project.
To address our questions, we focused on horned dung beetles from the tribe Onthophagini. In these beetles, the quality and quantity of nutrition received during larval development dictates whether males develop as large individuals with large horns or as small individuals with no horns. Large horns are used in male-male combat to gain access to females, while hornless males rely on more sneaky tactics to circumvent large males and intercept females. Importantly, like the castes of social insects horned and hornless males, too, constitute a polyphenism with very few intermediates seen in nature.
The wide diversity of horns and plastic responses found among Onthophagini allowed us to explore the mechanisms and evolution of plasticity. We focused on three species of horned beetles that show vast differences in the degree of plasticity: Onthophagus taurus, which exhibits an extreme, explosive response to nutrition; closely related Onthophagus sagittarius, which has secondarily lost horns; and distantly related Digitonthophagus gazella, which displays a modest plastic response, similar to what it is thought to be the ancestral condition for the tribe.

Left: Cattle farm where beetles and dung are regularly collected. Right: 12-well plate with D. gazella larvae
To collect tissues for transcriptomic analyses the first step is to get adult beetles from the field to start beetle colonies. Beetles are collected in organic cattle farms by our collaborators in North Carolina (O. taurus) and Hawaii (O. sagittarius and D. gazella), and then shipped to Bloomington, Indiana, where we keep them in separate colonies of ~300 beetles. Females build dung balls and lay a single egg in each ball. We collect the balls and open them to move the recently hatched larvae to be artificially reared in 12-well plates. This set up allowed us to manipulate nutrition levels by supplying dung of varying quality and pick either large, horned or small, hornless individuals of each species that were later used to dissect horn tissue and perform RNA extractions and sequencing.
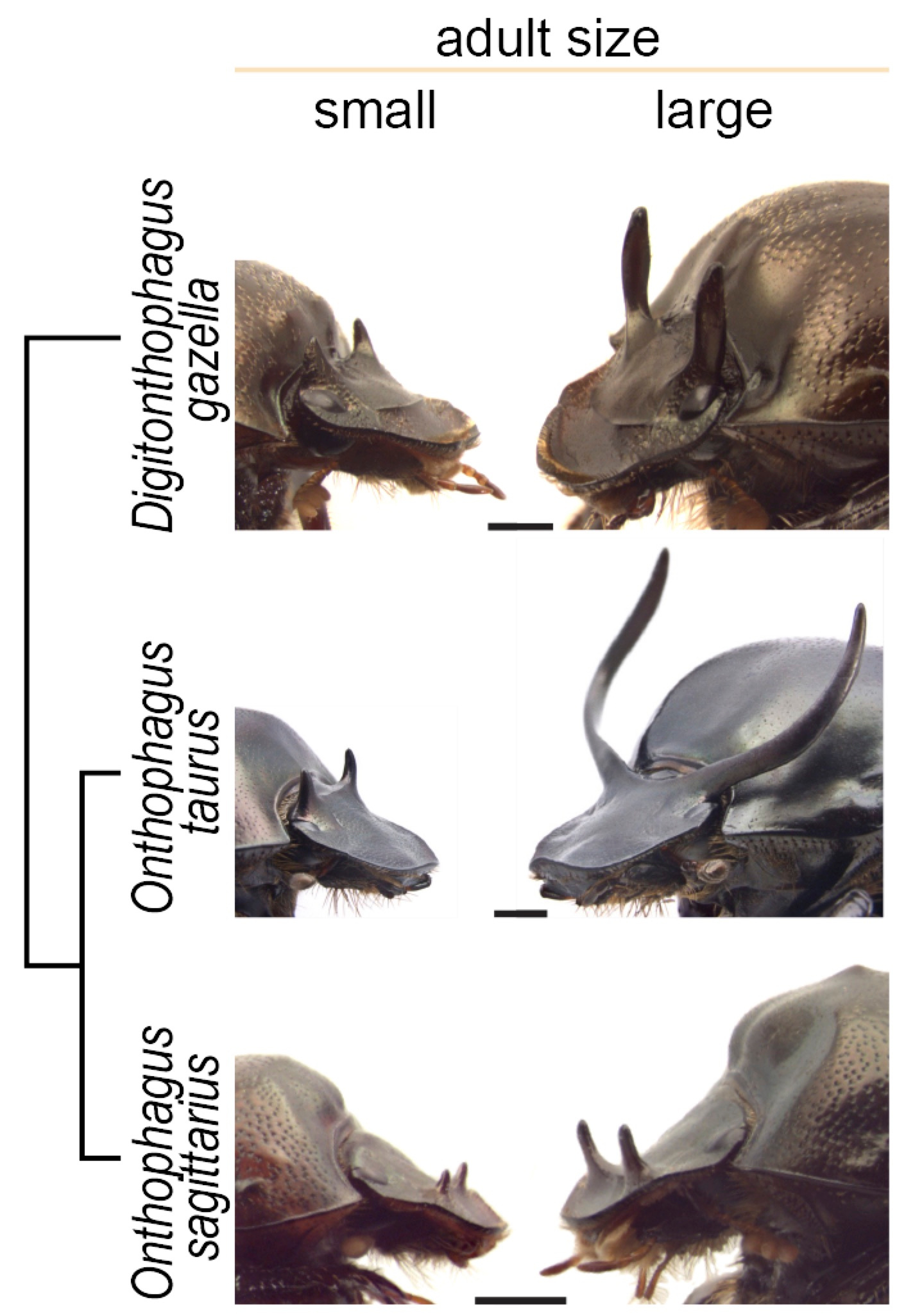
Small, low-nutrition males (left) and large, high-nutrition males (right) of the three species of beetle used in this study and their phylogenetic relationship.
We were particularly interested in understanding the relationship between gene expression changes across nutrition conditions and what we saw at the morphological level (horned vs hornless). Are differences in gene expression between small and large individuals greater in the species showing the more dramatic morphological response? How about the species that has lost the plastic response? Using RNAseq to estimate genome-wide expression in horn tissues, we found that gene expression differences mirrored what we saw at the morphological level; more exaggerated polyphenisms correspond with an increased number of nutrition-responsive genes. While this may seem intuitive, there was no previous evidence or expectation that this would be the case (e.g. we could have seen the same number of genes but more growth promoters vs. inhibitors in extreme responses).
Finally, we explored several additional questions that remain heavily debated in the field. For example, genetic accommodation is a process proposed to explain how variation in plastic responses among individuals can fuel evolutionary diversification of plasticity among species. Our data is consistent with such a scenario: ancestral plasticity in gene expression may have contributed to the evolution of growth exaggeration as well as loss in the Onthophagini tribe. Similarly, whether the evolution of novel degrees of plasticity is made possible by new genes (so called taxon-restricted genes) or facilitated by already existing, conserved, and re-purposed genes was unknown. We found evidence that a combination of both contributed to the exaggeration and loss of plasticity. As such our work advances our understanding of the evolution of developmental plasticity, a fundamental feature of most forms of life on our planet.
Follow the Topic
-
Nature Ecology & Evolution

This journal is interested in the full spectrum of ecological and evolutionary biology, encompassing approaches at the molecular, organismal, population, community and ecosystem levels, as well as relevant parts of the social sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Biodiversity and ecosystem functioning of global peatlands
Publishing Model: Hybrid
Deadline: Jul 27, 2026
Understanding species redistributions under global climate change
Publishing Model: Hybrid
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in