What it takes to grow liver cells
Published in Cell & Molecular Biology and Anatomy & Physiology

The liver is not only a critical organ executing many different functions to keep our body healthy, but it is also marked by a peculiar feature: its ability to extensively regenerate to replace damaged or lost tissue. Upon prolonged damage in chronic liver diseases, the regenerative capacity is ultimately reduced or lost. So, finding ways to boost the liver's capacity to regenerate holds great clinical promise.
In order to do so, we should first take a look at how liver regeneration normally works. Different liver cells can take on this role dependent on the setting and type of injury, however a key player is the main liver cell type: the hepatocytes. In mice, removal of two-thirds of the liver can be rebuilt into a full liver in a week, impressive!
If it is so easy in vivo, you would think that it would be easy to recapitulate this in vitro, right? Well... no. Since many years, researchers have tried to make human hepatocytes grow in a dish. Significant efforts have been made into the form of 2D cultures in which hepatocytes are allowed to dedifferentiate into a more stem/progenitor-like state. In this state, these cells can be significantly expanded and remarkably retain redifferentiation potential into hepatocyte-like cells (e.g. ref. 1).
In parallel, significant progress has been made to grow cells of the human liver in 3D format as organoids, which allows to recapitulate a higher level of tissue architecture and can translate to more mature cellular characteristics. In 2015, culture conditions were established to grow human cholangiocyte organoids (ref. 2-3). A few years later, different culture conditions were established to grow human hepatocyte organoids (ref. 4-5). However, the latter protocol was predominantly successful in growing fetal hepatocyte organoids, while attempting the same with adult hepatocyte organoids was much more difficult.
This led us to the question of our new study (ref. 6): what are the reasons behind the distinct growth behavior of fetal vs. adult human hepatocytes when attempting to establish 3D organoid cultures? To this end, we performed both transcriptomic and phenotypic approaches to provide time-resolved maps of the early mechanisms of organoid culture outgrowth, using both fetal and adult hepatocytes.
The results? Fetal hepatocytes initiate an inverse transcriptomic response between proliferation and lipid metabolism that allows the cells to grow. As the cells grow into bigger organoid structures, these responses return to baseline, yet when the organoids are allowed to grow again upon passaging the culture, the exact same mechanisms are put into place again. OK, so proliferation-metabolism is important to grow, but can we tweak the activity of the pathways to instead induce maturation? Yes! Removal of growth stimuli as well as boosting metabolic pathways resulted in the acquisition of more mature hepatocyte features in the fetal organoids. Learning from the transcriptomic data, we also discovered novel growth factors and cytokines that could boost the fetal organoid growth even further.
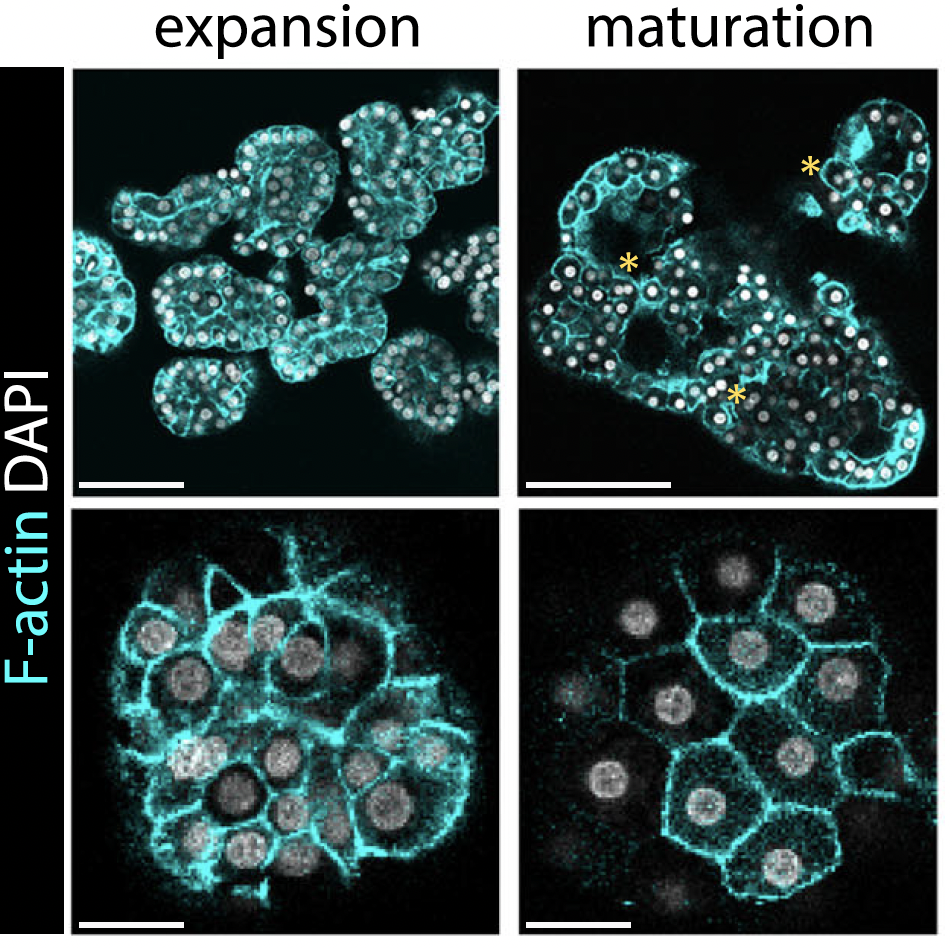
So what about the adult hepatocytes? While they initially mimicked the transcriptomic response as observed for the fetal hepatocytes, we noted that these signals quickly disappeared and organoid growth halted. What was more, the hepatocytes had acquired substantial amounts of lipids, adopting a state of a diseased liver. We then questioned whether the lack of a dynamic between proliferative and metabolic networks was the reason for the lack of growth. So, inspired by the growth signals we had discovered to work for the fetal organoids, as well as by recalling important metabolic hubs of the liver, in this case FXR, we devised a novel culture condition to make adult organoids grow better. We found that a combination of IL6 and FXR activation possessed a synergistic effect in boosting the adult organoid growth: the hepatocytes were healthy and kept growing for several months in culture. Interestingly, by performing single-cell RNA sequencing analyses comparing the fetal and adult organoids, we observed that "age identity" is a feature that is retained in organoid culture.
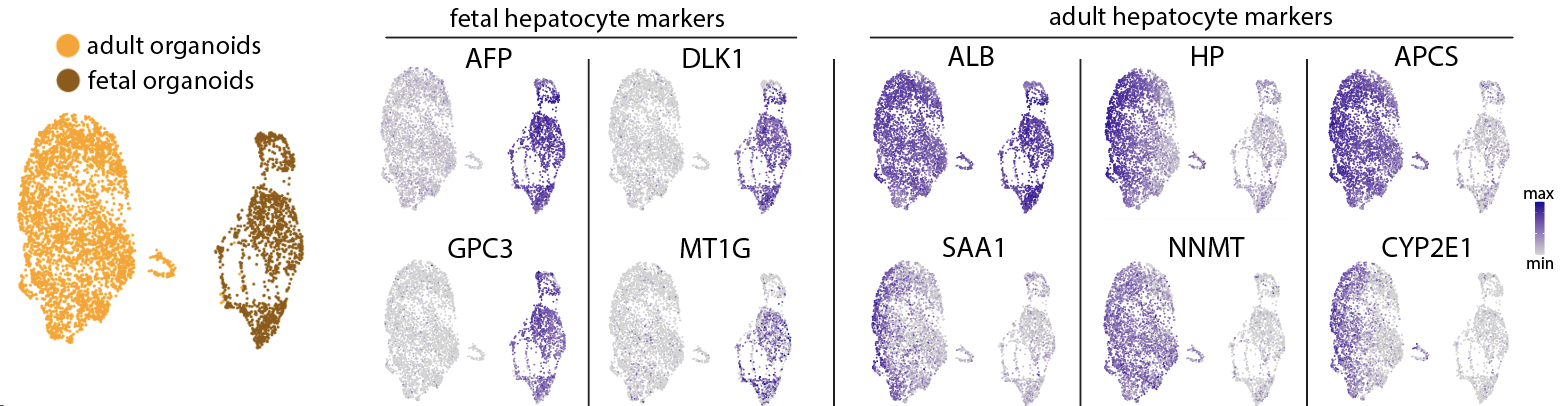
Our study not only provides optimized culture conditions to grow and mature hepatocytes, but it also reveals novel insights into hepatocyte behavior. We find that hepatocytes in adulthood become restricted and more specific in their needs to grow, while instead in development hepatocytes are much more "pan-responsive". This thus suggests that a very specific cocktail of molecules may be needed to unleash the regenerative power of an older liver. Our identification of the synergy between IL6 and FXR activation to boost hepatocyte growth also makes us question whether these findings could hold translational power. Would this combination of molecules work in mouse models, and can it hold potential also in different liver disease scenarios? We hope to find answers to some of these questions in our future research.
References
1. Zhang et al. Cell Stem Cell, 23(6):806-819.e4, 2018.
2. Huch et al. Cell, 160(1-2):299-312, 2015.
3. Broutier et al. Nat Protoc, 11(9):1724-43, 2016.
4. Hu et al. Cell, 175(6):1591-1606.e19, 2018.
5. Hendriks*, Artegiani* et al. Nat Protoc, 16(1):182-217, 2021.
6. Hendriks*, Artegiani* et al. Nat Commun, 2024, doi: 10.1038/s41467-024-48550-4.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in