When organic red single-crystal becomes black: narrowing band gap energy chemically and with application of physical pressure
Published in Materials

The research group in the Center for High Pressure Science & Technology Advanced Research (HPSTAR) love to compress elements, alloys, and molecules. High-pressure apparatus, such as diamond anvil cell (DAC) and large volume press, are used for carrying out researches in various scientific fields. Creating and enabling access to the unknown state of matter under extreme conditions.
In particular, DAC is extremely useful in conducting spectroscopic experiments while applying a large amount of force on a small amount of area. This is possible since the diamond is transparent, the hardest material and virtually incompressible, making it easy to observe the samples status as they are being compressed during experiments. With significant development of high-pressure experimental techniques in recent years, a lot of excitement were built in the research of “light elements under ultra-high pressures”. Technically, DAC can generate tremendous net pressure, millions of atmospheric pressures, as seen in a study published in Nature, that maybe even enough to make hydrogen into metallic state.(Nature, 2002) Although, an unambiguous experimental confirmation of such observations remain extremely difficult. Instead, recent efforts have focused on hydrogen-dominant alloys such as binary and ternary metal hydrides, in which the electron density at the hydrogen atoms is pre-compressed comparable to pure hydrogen compressed to megabar pressure.
Can we observe something as extraordinary like that in carbon as well?
I have been investigating carbon based molecular compounds from the days in Master degree project, and I joined HPSTAR thinking I should be able to observe something extremely interesting under high pressure. In theory, carbon-rich materials are also promising candidates, similar to hydrogen-rich materials, as they can provide the high-frequency phonon modes and strong electron-phonon coupling necessary for achieving high Tc superconductivity. A recent theoretical study highlighted that benzene (C6H6) - hydrogen and carbon “alloy” - can effectively decrease its band gap by applying high-pressures to enhance electron-phonon interaction. This mechanism can also induce a transition into a metallic state in a narrow pressure range between 180-200 GPa. (J. Am. Chem. Soc. 2011)
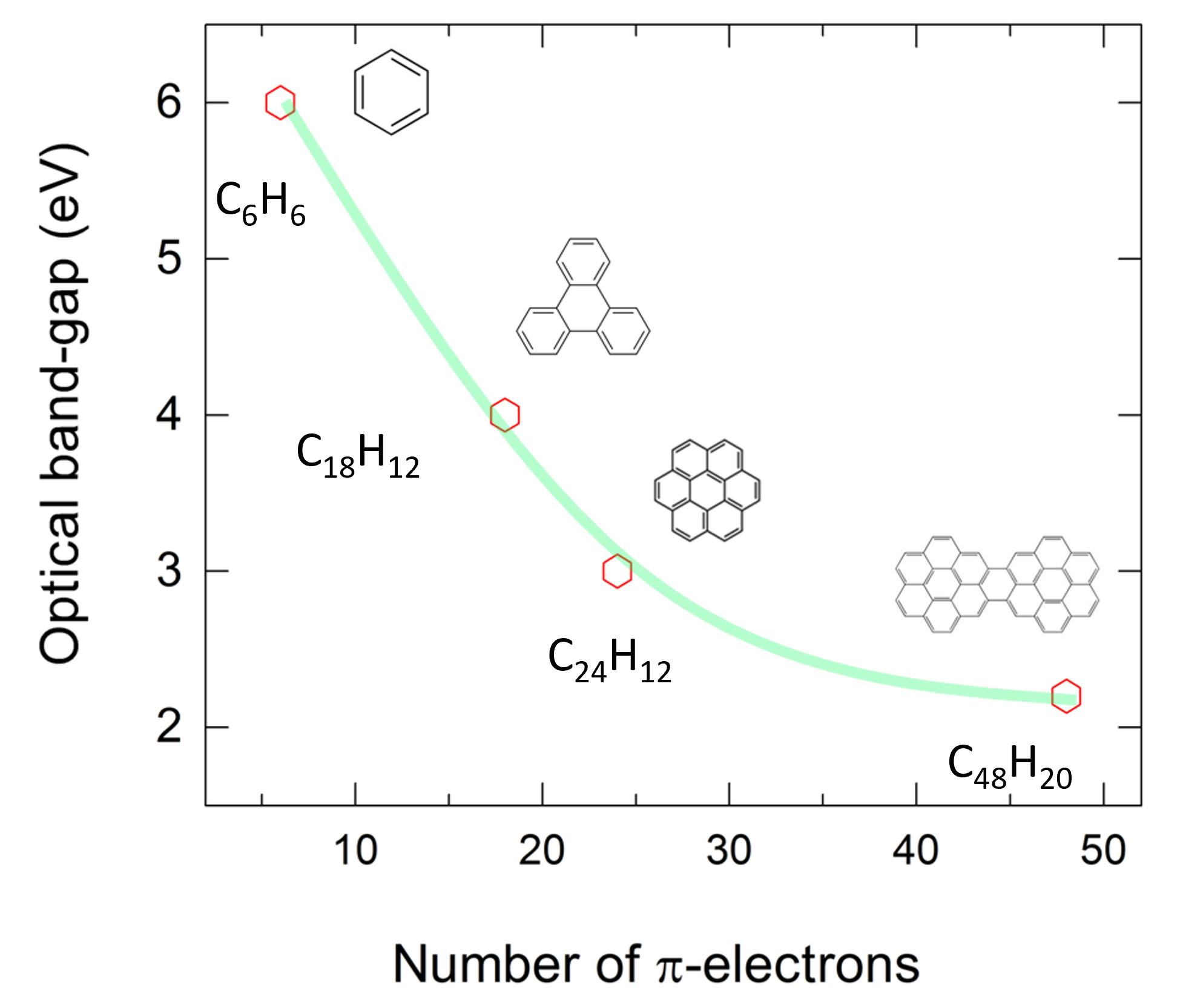
Reviewing the experimental data, high-pressure studies involving conjugated organic molecules such as polycyclic aromatic hydrocarbon (PAH) solids above 10 GPa are scarce. This is largely down to the fact that organic molecular crystals are easily decomposed when compressed and the technical difficulties of conducting tests on such delicate materials. In this research, instead of finding a way to apply 200 GPa on C6H6, we combined “chemical compression” and “physical compression” on PAH molecules to reduce their band gap energy effectively and efficiently. More specifically, the band gap energy of PAH molecules can be reduced by increasing the number of π-electrons, which can be defined as chemical pre-compression. For instance, the band gap energy of C6H6 at ambient condition is 6 eV, which can be reduced down to 2.2 eV by increasing the number of benzene rings to form dicoronylene (C48H20). (Figure 1) Then, physical compression is applied using diamond anvil cell (DAC), where the distance between adjacent molecules will be reduced, enhance intermolecular interaction, and leads to narrowing band gap energy.
For this project, researchers from HPSTAR, Ruđer Bošković Institute, University of Edinburgh, National Synchrotron Radiation Research Center, and European Synchrotron Radiation Facility teamed up to investigate thoroughly the properties and structure evolution under high pressure. We observed a continuous closing of the band gap energy from 2.2 eV down to 0.7 eV at 33.6 GPa, accompanied by a change in the optical color of the crystal from red to completely black. Electronical resistance measurements conducted as a function of P/T suggest that the semi-metallic character of C48H20 becomes apparent in the pressure range of 23.0-38.0 GPa. High-pressure Raman spectroscopy measurements indicated the possible structural transition above 23.0 GPa. The crystallographic information at high-pressure was the last most challenging part of this study. As far as we know, no other single-crystal data on PAHs have been reported to date that provide meaningful information for interpreting predictions and understanding the relationships between their structure and properties.
We carried out both powder and single-crystal X-ray diffraction measurements at synchrotron facilities, SPring-8 (BL12b) and ESRF (ID15b), respectively. The crystallographic information on the complete structure solution and refinement were successfully obtained at various pressures. (Figure 2) All structures could be solved evidencing the monoclinic space group P21/c. At 26.2 GPa, the unit cell volume was reduced by 20.0% compared to ambient pressure. The intermolecular distances were also calculated on the basis of the experimental data. In particular, the C-C distance between the atoms of the nearest-neighbour molecules were reduced from 3.474(3) Å at ambient pressure to 2.724(7) Å at 26.2 GPa. The data suggest that the semi-metallic state occurs when the intermolecular C-C distance is shorter than 2.8 Å and the irreversible chemical reaction takes place at 2.6 Å. The electron localization function obtained based the structural information show that the van der Waals space between C48H20 molecules decreases due to the compression of the intermolecular distances and the rotation of the molecules.
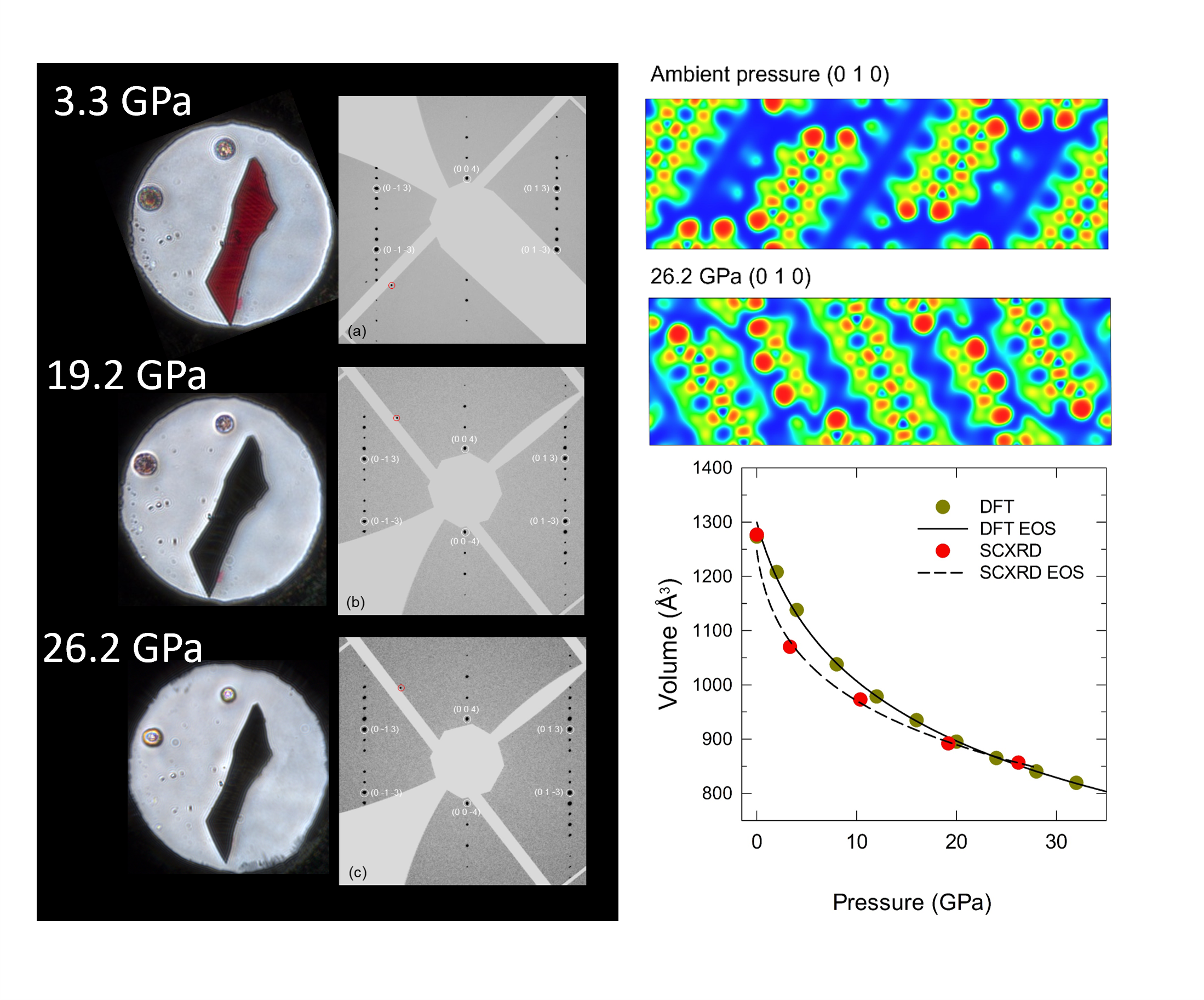
Fig. 2: Single-crystal under high pressure (left) Optical micrograph and (0kl) reciprocal space images generated from SCXRD experiments on dicoronylene (C48H20). (right) The charge density distribution and the P-V equation of states based on high-pressure experiments.
We studied the pressure-induced physical features in a compound consisting of only carbon and hydrogen and observed a tuneable band gap, altered electric conductive behaviour, providing evidence for the semi-metallic nature and bridging the structure-property relationship in C48H20. This single-component, single-phase single crystal of pre-compressed large PAH molecules is stable under ambient conditions and may be thoroughly characterized prior to the application of physical compression. The pressure responses of the other large PAH molecules to a possible transition to the metallic and superconducting state should be further investigated in the light of our findings.
Feel free to explore the complete study on dicoronylene under high-pressure at: https://doi.org/10.1038/s43246-025-00814-2
Follow the Topic
-
Communications Materials

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of materials science.
Related Collections
With Collections, you can get published faster and increase your visibility.
Advanced characterizations of high-entropy materials
Publishing Model: Open Access
Deadline: Mar 31, 2026
Multifunctional hydrogels
Publishing Model: Open Access
Deadline: Feb 28, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in