Widespread co-release of glutamate and GABA throughout the mouse brain
Published in Neuroscience
https://www.nature.com/articles/s42003-024-07198-y
This research began with an intriguing observation of distinct electrical signals in mouse brain synaptic recordings, known as “biphasic minis”. Our research builds on Shabel et al.'s (2014) pioneered observation of biphasic minis from the lateral habenula. We expand this finding by investigating these unique signals throughout the entire brain.
Synaptic neurotransmitter release typically occurs in response to an action potential but can also happen spontaneously, producing miniature currents, or “minis.” These smallest detectable synaptic signals result from a single vesicle releasing its contents. While neurons can release multiple neurotransmitters, whether this involves separate vesicles or one containing both is debated.
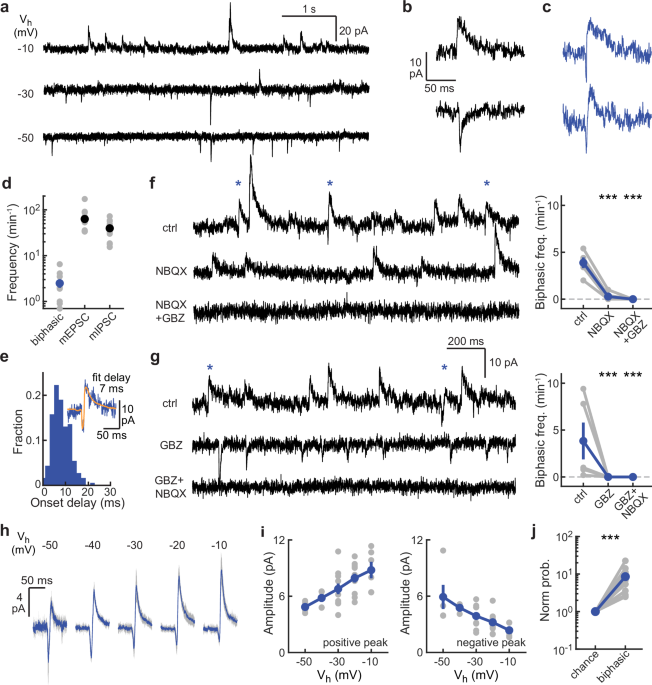
To explore this question, we performed electrophysiological intracellular recordings using the patch-clamp technique and analyzed synaptic signals that appeared to involve simultaneous release of glutamate and GABA. In standard mini recordings, such signals would look like a combination of excitatory (miniature excitatory synaptic currents or mEPSCs) and inhibitory currents (miniature inhibitory synaptic currents or mIPSCs). Due to the slower kinetics of the GABAergic current, this combined signal displays a characteristic downward and upward phase, hence the term “biphasic”.
Occasionally, two closely timed currents (one mEPSC and one mIPSC) may appear together by chance, based on the joint probability of these two events occurring within a specific time window, expected to be shorter than the kinetics of synaptic currents. Surprisingly, we found these events occurring at a much higher frequency than expected, suggesting a synaptic mechanism for time-correlated release of glutamate and GABA. Our findings provide evidence that biphasic minis indeed correspond to tightly synchronized mEPSCs and mIPSCs within a very short time frame, close to 10 ms.
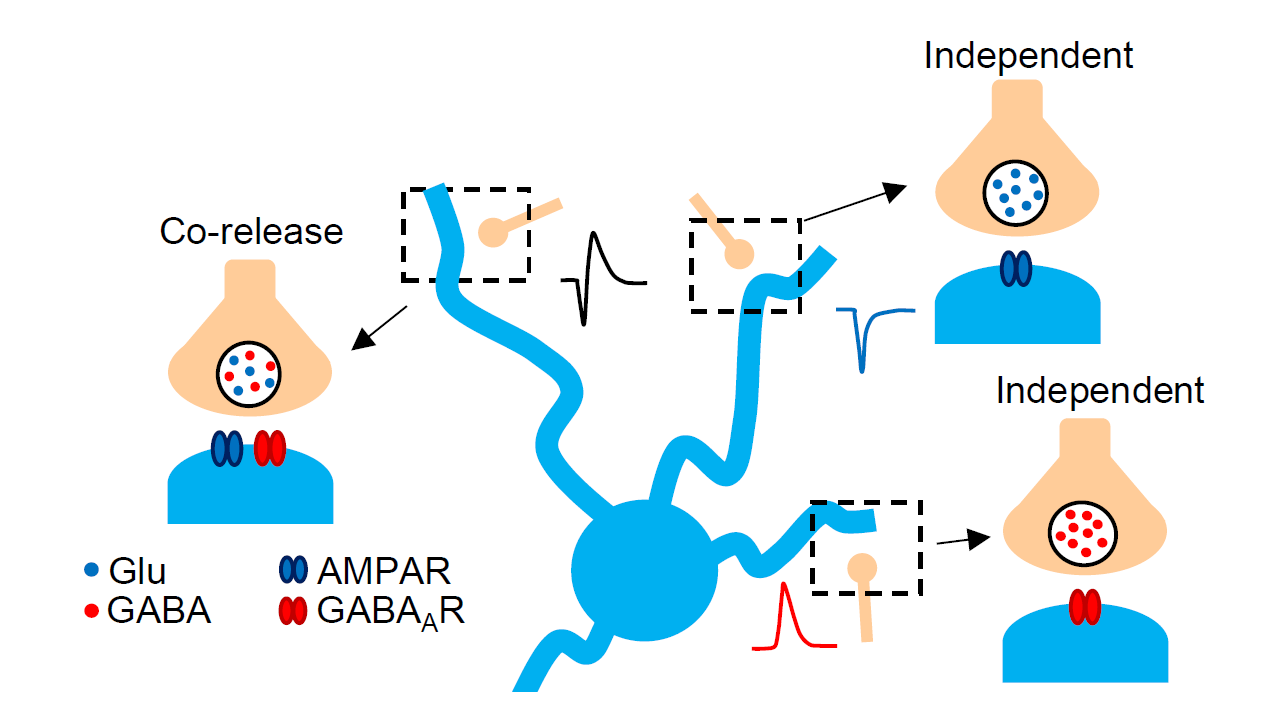
Interestingly, we observed two types of biphasic minis: one with an mEPSC followed by an mIPSC (a “straight” biphasic mini) and another with an mIPSC followed by an mEPSC (a “reverse” biphasic mini). We propose that both patterns may result from different synaptic architectures, where AMPA or GABA receptors are positioned closer to the release site.
Initially, we focused on neurons in the striatum of the mouse brain. To explore whether biphasic minis represent a broader synaptic mechanism, we extended our recordings to other brain regions across the entire mouse brain. Interestingly, we found highly frequent straight biphasic minis in nine out of ten regions and reverse biphasic minis in seven out of ten, suggesting that co-release of glutamate and GABA may represent a widespread synaptic mode in the mouse brain.
We hope our study will encourage further exploration of this phenomenon, i.e. the co-release of glutamate and GABA from the same synaptic vesicles. An important next step will be to identify neuron populations capable of co-releasing glutamate and GABA throughout the mouse brain, which, according to our findings, are likely to have widespread axonal projections.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in