Regulation of Human Genome Editing in Singapore
Published in Social Sciences, Genetics & Genomics, and Public Health
Please refer to the following journal articles:
Stringent criteria needed for germline genome editing of human IVF embryos
Genome Editing Should Preferably Be Carried Out on Fetuses In Utero Rather Than IVF Embryos
Do not overlook the possibility of genome-edited somatic cells ending up in the human germline
Please also listen to the audio podcast:
Key aspects of how human genome editing should be regulated include:
-
Establishing International Regulatory Frameworks and Oversight
◦ International bodies like the World Health Organization (WHO) should establish standing committees to draft rigorous and comprehensive international regulatory frameworks.
◦ These frameworks must provide strict oversight on human genome editing for both experimental and therapeutic applications.
◦ Particular tight governance should be imposed on the transmission of genetic modifications to the human germline.
◦ Regulators at all levels need to actively review and adapt current ethical guidelines and laws to keep pace with scientific advances, formulating clear and unambiguous directives.
◦ The international regulatory framework must specifically account for the plausible chance of genome-edited somatic cells ending up in the human germline, whether intentionally or inadvertently, through various new technology platforms.
-
Principles for Application and Decision-Making
◦ Regulations should be based on core medical ethics principles, including non-maleficence (minimizing harm) and autonomy (ensuring informed consent).
◦ Rigorous and comprehensive counseling must be provided to prospective parents with known genetic mutations, enabling informed consent and autonomous decision-making when choosing between fetal or embryo genome editing. This counseling must cover pertinent safety risks and available alternatives.
◦ There must be a clear distinction in future legislation between preventing genetic diseases and human enhancement. Recognizing that these boundaries can be blurred, intensive dialogue among medical doctors, scientists, bioethicists, and lawmakers is essential for delineation and clarification.
◦ Regulations should consider sociocultural factors in different countries, where alternatives like gamete/embryo donation or child adoption may not be culturally acceptable. In such cases, germline genome editing of IVF embryos might be permitted under strictly limited and tightly regulated conditions, as a last resort.
-
Regulation of Germline Genome Editing of IVF Embryos
◦ This type of editing is controversial because it is not directly health or lifesaving, but rather intended to prevent genetic diseases in future offspring, and carries substantial medical risks.
◦ Stringent criteria are proposed for future clinical trials, primarily based on the principle of non-maleficence:
▪ It should be cautiously and judiciously applied, avoiding non-essential usage.
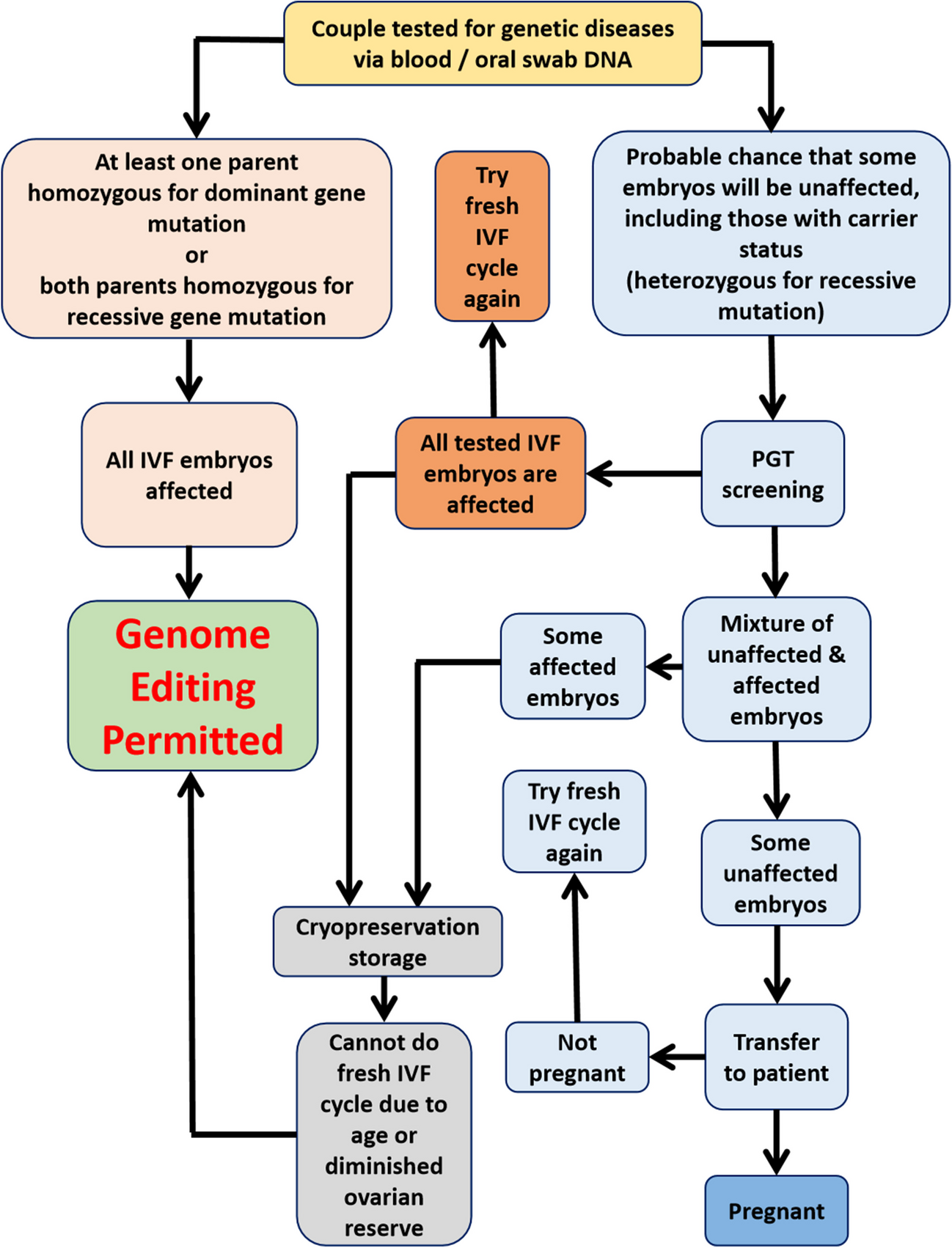
▪ If there is a fair chance that some IVF embryos will not be affected by genetic diseases, initial preimplantation genetic testing (PGT) screening must be performed to identify unaffected embryos for transfer.
▪ IVF embryos with carrier status (heterozygous for a recessive gene mutation) should not undergo germline genome editing to avoid unnecessary risks, as they can still result in an unaffected child.
▪ If patients fail to conceive after transferring unaffected embryos, they should be encouraged to undergo another fresh IVF cycle rather than opting for genome editing of remaining affected embryos.
▪ Genome editing of remaining affected embryos should only be permitted as a last resort, if the patient is unable to produce any more unaffected embryos in a fresh IVF cycle due to advanced maternal age or diminished ovarian reserves.
◦ There should be rigorous pre-clinical data from multi-generational animal studies to better understand the effects on future generations before such procedures are widely adopted.
◦ Worldwide regulatory bans should be imposed on the utilization of mosaic chimeric preimplantation embryos in clinical assisted reproduction due to increased risks of cancer and autoimmune diseases. Regulations should specify that genome-edited somatic cells or their induced pluripotent stem cell (iPSC) derivatives can only be used for gene therapy past a specific developmental stage beyond preimplantation embryos, to minimize germline infiltration.
-
Regulation of Fetal Genome Editing In Utero
◦ Fetal genome editing is considered less ethically contentious than embryo genome editing because it typically does not involve man-made genetic modifications transmitted to future generations.
◦ However, concerns exist that genome editing performed on fetal somatic cells in utero may unintentionally transmit to germline tissues due to the high plasticity and extensive tissue remodeling during fetal gestation.
◦ This possibility necessitates rigorous postpartum genetic screening and testing of offspring of fetal gene therapy. However, current methods for detecting genome-edited cells in the germline may require invasive and potentially destructive biopsies for sexually mature females.
◦ Amendments to relevant healthcare regulations may be required to enable patients to opt for genome editing of fetuses in utero as an alternative therapy to IVF embryo editing, especially if restrictive legislation on transferring affected IVF embryos remains in place.
◦ Genome editing of surgically-extracted fetal somatic cells followed by subsequent re-transplantation back to the fetus in utero may represent a better strategy for avoiding heritable genome editing errors and unknown detrimental effects.
Follow the Topic
-
Journal of Assisted Reproduction and Genetics

This journal publishes cellular, molecular, genetic, and epigenetic discoveries advancing our understanding of the biology and underlying mechanisms from gametogenesis to offspring health.
Related Collections
With Collections, you can get published faster and increase your visibility.
Outcomes in ARTs
Publishing Model: Hybrid
Deadline: Ongoing
Unlocking the potential of artificial intelligence (AI) in reproductive medicine: The JARG collection on assisted reproductive technology (ART) and machine learning
This collection of papers brings together the latest, cutting-edge research and delves into the promising role of Artificial Intelligence (AI) in shaping the future of reproductive medicine. With an aim to inspire and ignite innovation, this collection invites authors to contribute their own original and cutting-edge research, while providing readers with an exciting array of advancements in this rapidly evolving field.
Artificial intelligence surrounds us in daily living. But in an indirect, non-clinical and arm’s length way. And as such a devil-may-care attitude abides. If it works, who cares about details? (Most of us can't tell the difference between Python, Java, or C++ and frankly don’t care). It’s simply not part of the daily to-and-fro of delivering assisted reproductive technologies (ART). But evidence is mounting that AI can provide additional horsepower to the engine of decision making in ART. And this migration of AI into the front yard of clinical care should prompt us all to sit up, pay attention and take notes. Like it or not, AI has arrived on the ART scene. New AI tools are rapidly emerging in ART but without an easy-to-read user’s manual. As with all things new and novel, education is the foundation to make smart and well-informed decisions and avoid making premature grab-and-go choices in response to a hot topic pitch that we may live to regret.
As highlighted in this collection, the potential of AI in the reproductive space is spectacular, enabling more accurate predictions of pregnancy outcomes, optimized embryo selection, and personalized treatment plans based on individual characteristics and genetic profiles. Harnessing the power of AI, reproductive medicine is undergoing a paradigm shift, redefining the boundaries of what is possible. Machine learning algorithms have paved the way for improved diagnosis, treatment, and patient care. By leveraging vast amounts of data, AI systems can identify patterns, make predictions, and provide tailored recommendations to healthcare providers and patients.
However, as always, progress also comes with perils. Our field is ripe with examples of the principle “implementation before validation” where the enthusiasm for the utilization of new technologies exceeds the appreciation for potential pitfalls and risks. This phenomenon appears to be almost inevitable in the case of artificial intelligence (AI) assisted reproductive technology (ART). There is no longer any doubt regarding the integral role that AI will play in ART. While the “if” question regarding implementation is answered, we can still control the “how.” Are we outsourcing clinical care to a computer? How do AI tools impact the quintessentially clinical process that, until now, was solely within the purview of providers? Solid, high-quality prospective studies are needed to demonstrate improved outcomes and ideally, cost reduction. The crossroad between biology and informatics needs to be traversed by academics and experienced clinicians, as well as statistical experts, and guarded by solid peer review, rather than occupied entirely by commercial interests. Data integrity and safety are paramount. Furthermore, the care of our patients should continue to be holistic, empathetic, and not de-humanized. AI should not just provide fancy gadgets for to the privileged few, but advantages to the reproductive patient collective as a whole.
This collection showcases a wide range of papers that investigate the profound impact of AI on fertility-related topics, including assisted reproductive technologies, reproductive health monitoring, predictive modeling, and personalized medicine. By featuring a diverse range of interdisciplinary research, this collection serves as a platform for scientists, clinicians, and engineers to share their insights, innovations, and future visions. Through collaboration and knowledge exchange, we aspire to accelerate the progress of AI in reproductive medicine, ultimately improving outcomes for people struggling with infertility and reproductive health challenges.
Join us in this stimulating journey through the cutting-edge science of AI and fertility. Explore the remarkable breakthroughs, be inspired by the endless possibilities, and contribute your own original research to shape the future of reproductive medicine. Together, we can push the boundaries of science, technology, and human potential to revolutionize the field of fertility and bring hope to millions around the world. Read on and make your own decisions about how these new tools can positively impact what we do as individual practices and as a specialty. As we enter this new era, in awe of the rapid breakthroughs occurring in front of our eyes, we continue to remind ourselves of the ultimate goal of our work: healthy babies.
Publishing Model: Hybrid
Deadline: Ongoing





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in