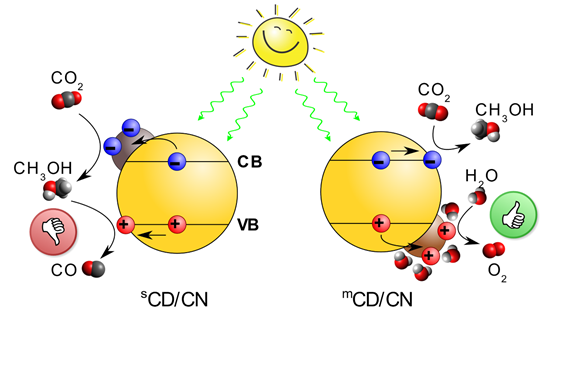
Methanol, a liquid fuel alternative to petrol, could now be sustainably produced via a carbon-neutral pathway using only sunlight, greenhouse gas CO2 and water with unity selectivity! By careful design and integration of carbon-dots/carbon-nitride (CD/CN) junction, we show the stable conversion of water and CO2 to stoichiometric O2 and methanol with nearly 100% selectivity under sunlight. The key behind these new exciting results is the unique hole-accepting nature of the novel crystalline CD and its selective adsorption of water over methanol.
Two photoreactions are driven by the system: 1) water (H2O) oxidation and 2) CO2 reduction to high-value products, in this case, methanol (CH3OH). High-performance photocatalysts utilise co-catalysts to enhance the catalytic properties of the surface and accelerate the rate of reaction. The heterojunction between the light-absorbing photocatalyst and the co-catalyst also makes electrons and holes flow to different materials and limit how fast they find each other (leading to the annihilation reaction termed recombination). This translates to an increase in the lifetime and a greater chance they do the desired reaction.
There is seemingly a tendency to focus on the reduction reaction co-catalyst as this produces the high-value product (think methanol from CO2 or H2 gas from protons). However, the water oxidation reaction is usually the slowest step and will limit the efficiency of the whole system. The development of water oxidation co-catalysts that can extract holes from the photocatalyst and promote reactivity is a more direct path to an improved system.
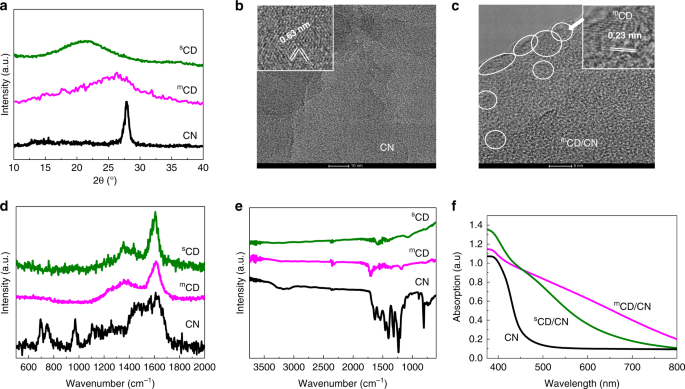
This is exactly what we found for mCD, fabricated via microwave-assisted method. Through time-resolved spectroscopic investigations on our carbon-based nano architectures, we observe that sCD, synthesised from a traditional sonication method, work as an electron acceptor in sCD/CN. Remarkably, mCD serves as a notable hole acceptor in mCD/CN. Charge separation across the CD/CN interface increases the lifetime of the charge carriers from 25 µs (CN) to 160 µs (mCD/CN) or only 40 µs (sCD/CN). The longer lifetime in mCD/CN enhances participation in subsequent reactions.
The differences in photophysical functions of the CDs result in the differences in both conversion efficiency and more importantly, selectivity. sCD/CN junction converts CO2 into CO while the mCD/CN junction could selectively reduce CO2 to methanol with unprecedented selectivity of 99.6±0.2 %. Furthermore, the mCD/CN composite is 12 times more active than sCD/CN for CO2 conversion observed under the same experimental conditions.
Computational results showed that water prefers adsorption to mCD (where holes accumulate) while methanol prefers adsorption to CN (where electrons accumulate), thus facilitating selective oxidation of water to O2 while avoiding unproductive oxidation of the photoreduction product, methanol. The production of methanol proceeds with an exceptional near unity selectivity and high IQY of 2.1% at 420 nm and benchmark IQY at 500 nm and 600 nm (0.7% and 0.4%, respectively).
This strategy selectively reduces greenhouse gas by water to high-value chemicals, hence offering sustainable liquid fuels and closing the global carbon cycle. For more details on this study, please see our recent article [link] “Unique hole-accepting carbon-dots promoting selective carbon dioxide reduction 100% to methanol by pure water” in Nature Communications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in