Assessing Feasibility of Mobile Technologies Prior To Implementation in Clinical Research
Published in Healthcare & Nursing
Using mobile technologies such as wearables for data capture in clinical trials has the power to transform the clinical research enterprise by increasing efficiency, reducing costs, facilitating remote data capture, and enabling the collection of more relevant and meaningful real-world data. However, with new options entering the market daily, it can be difficult to know which solution to use for best results in a particular clinical trial.
In an effort to address this challenge, the Clinical Trials Transformation Initiative (CTTI) recently developed a set of recommendations around optimal use of mobile technologies for data capture in clinical trials. Through this work, we found that conducting a technology-focused feasibility study prior to launching a full-scale clinical trial is a critical step in order to reduce risk and avoid costly mistakes. As suggested in CTTI’s recommendations, feasibility studies are an effective way to enhance participant experience, evaluate technology performance, assess data quality and security, identify possible logistical hurdles, and address other unanticipated issues within the context of a specific trial.
We also recognized the potential for duplication and decreased efficiency if researchers were to run overlapping feasibility studies independent of one another. As the adoption of mobile technology in clinical research increases, we saw the need for a central location where feasibility studies could be easily accessed, enabling researchers to explore how various technologies have been utilized and assessed in prior feasibility studies.
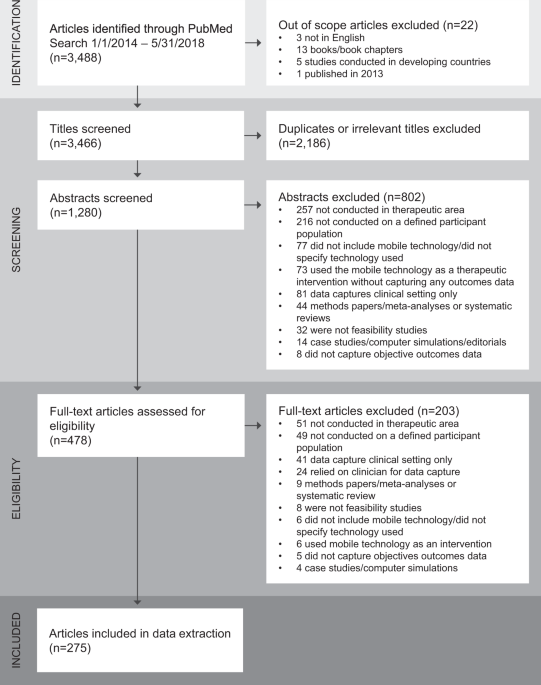
The CTTI Interactive Database of Feasibility Studies for Mobile Clinical Trials was born from this need. The database is the first publicly available resource that contains a catalogue of published feasibility studies related to the use of mobile technologies in clinical research.
This new tool presents many opportunities. In addition to encouraging collaboration and reducing redundancy, the database will help optimize the use of wearables and other devices in clinical research by encouraging standardization, and demonstrating the value of publishing these important studies. Stakeholders can learn about different facets of mobile technologies, such as sensor performance and algorithm development, from the findings of more than 275 studies. Users will be able to filter and search by therapeutic area, technology type, participant characteristics, and a host of other variables.
To keep pace with a rapidly-advancing field, CTTI plans to regularly maintain and update the database so that users can easily access the most current feasibility studies. Users can also submit a paper for possible inclusion in the database. Ultimately, CTTI hopes the database will help researchers reap the full benefits that mobile technologies have to offer. https://rdcu.be/bFIkR
Follow the Topic
-
npj Digital Medicine

An online open-access journal dedicated to publishing research in all aspects of digital medicine, including the clinical application and implementation of digital and mobile technologies, virtual healthcare, and novel applications of artificial intelligence and informatics.
Related Collections
With Collections, you can get published faster and increase your visibility.
Digital Health Equity and Access
Publishing Model: Open Access
Deadline: Mar 03, 2026
Evaluating the Real-World Clinical Performance of AI
Publishing Model: Open Access
Deadline: Jun 03, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in