A Convenient CRISPR-Cas9 Conjugation Platform Allows for Beta Cell Engineering
Published in Bioengineering & Biotechnology

CRISPR-Cas9 has been used for a plethora of applications including biomaterials, cell engineering, and therapeutics, to name a few.1, 2 However, some applications of this technology are currently limited, partly because prolonged Cas9 activity can cause severe off-target effects. Thus, our lab focuses on ways to precisely control Cas9 so that it is activated at the right time and in the right location to maximize its potential.3 Previously, we demonstrated Cas9 activation4-6 and inhibition using small-molecule triggers.3, 5, 7 In our recent Nature Communications paper,8 we added to the toolbox of Cas9 by developing a convenient CRISPR-Cas9 conjugation platform that can even further expand the applications of this technology.
In gene editing, Cas9 breaks the DNA chains, which is then repaired using one of two pathways: either non-homologous end-joining (NHEJ), which can lead to uncontrolled results, or the more desirable the homology-directed repair (HDR) pathway that bases the repair off of a supplied template single-stranded oligonucleotide donor DNA (ssODN). Because the chances of biasing repair towards the desired pathway are higher with a local supply of the template DNA (ssODN), we wanted to devise a simple way to tether ssODNs or any cargo to Cas9.
Two key benefits arise from conjugation of an ssODN to Cas9. First, conjugation of the ssODN to Cas9 takes a three-component system (ssODN, Cas9/gRNA, and target DNA) to a two-component system (ssODN-Cas9/gRNA and target DNA). As with chemical reactions in a flask, a three-component reaction has a lower probability to proceed as compared to a two-component system. Second, a free-floating biomolecule, such as a highly negatively charged ssODN, is typically less stable than one attached to a protein.
We devised key objectives for our tethering platform. Ideally it should be (1) amenable to a diverse set of cargoes, (2) robust and easy to use by non-specialists, (3) high yielding to accommodate expensive cargos, (4) inexpensive, and (5) scalable. As a result, we employed the robust thiol-maleimide conjugation system, which allowed for tethering to internal sites of Cas9 (as compared to other studies that attached ssODNs to the N- or C-terminus) as well as for the multivalent display of these cargoes (Fig. 1 below).
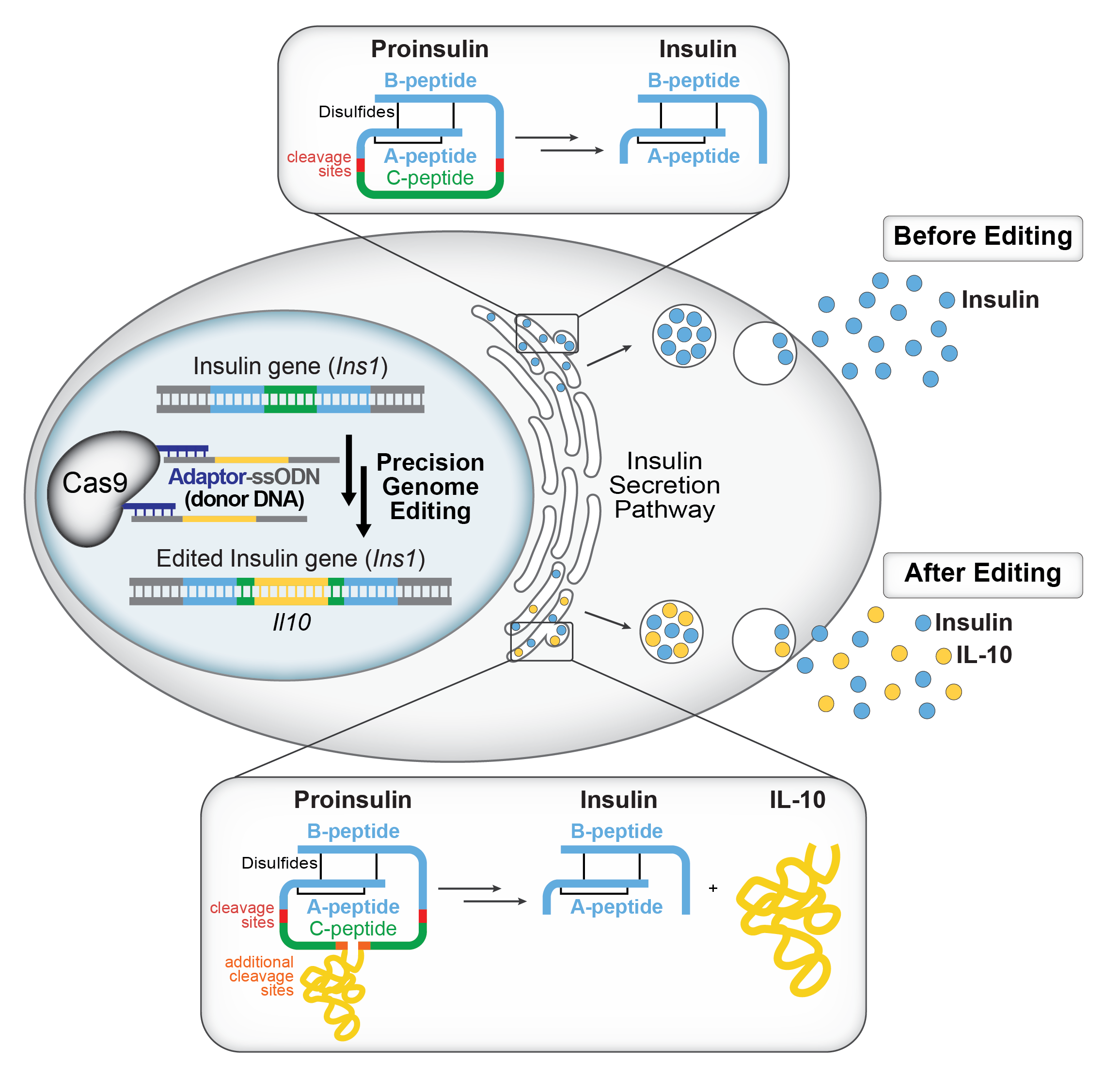
Figure 1. Our developed technology allows for Cas9 to display various cargoes. The mono- or multivalent adaptor sequence allows for ssODNs containing a small (17 nt) complementary DNA sequence to be tethered to Cas9 to enhance HDR in a variety of cell types. We can knock in Il10into the insulin gene (Ins1) at the C-peptide so that when insulin is processed, insulin and anti-inflammatory IL-10 are secreted together. Top box:When endogenous insulin is processed, the C-peptide is cleaved and insulin is secreted. Bottom box:When our edited insulin is processed, the C-peptide is cleaved and both insulin and IL-10 are secreted. We envision that these designer beta cells will propel cell replacement therapeutics for Type 1 diabetes, and that the gene editing technology will be applied to other system to enhance HDR.
We engineered cysteine sites throughout Cas9 to conjugate a 17-nt adaptor oligonucleotide using maleimide chemistry. The resulting Cas9-adaptor conjugates were readily hybridized to diverse ssODNs to give Cas9-ssODN conjugates. We used these conjugates for enhancing knock-in rates in several cell types (hiPSC, primary fibroblasts, U2OS, MDA-MB231, HEK-293FT), at diverse genomic loci (GAPDH, PPIB, CFL1), and with diverse knock-in fragments (HiBiT, GFP11, and several base-exchange fragments; Fig. 2 and 3 of the paper).
Moreover, we were able to identify which sites were best for conjugation. While the monovalent display showed enhanced knock-in efficiencies with the Cas9-adaptor compared to untethered Cas9, we then investigated a multivalent display of the ssODNs by using two cysteines. Here, we observed an even bigger increase in knock-in efficiencies (Fig. 4 of the paper).
A substantial motivation behind developing this technology was for the application of diabetes. Previous studies revealed that IL-10, an anti-inflammatory cytokine, has been shown to enhance long-term beta-cell survival and reduce fibrosis in transplanted beta cells in diabetes patients.9 We hypothesized that if both insulin and IL-10 are secreted, it could greatly help cell-based therapeutics for diabetes (Fig. 5 of the paper). However, to make a cell secrete a foreign substance is extremely difficult.
We were inspired by previous work that demonstrated that luciferase could be engineered into the C-peptide of insulin,10 and so we hypothesized that if we inserted Il10using our Cas9-adaptor system, we could secrete insulin along with IL-10. We first knocked in a short HiBiTsequence to identify the optimal knock-in site on the C-peptide, and then successfully knocked in Il10(480 nt knock-in fragment) into Ins1in INS-1E beta cells (Fig. 6 of the paper). We hope that this engineered glucose-stimulated IL-10/insulin secretion in beta cells will serve as a potential therapeutic for T1 diabetes patients (Fig. 1 above).
Unlike other conjugation methods, our work uses small nucleotide fragments (versus proteins), can append these sequences to internal sites on Cas9 (versus C- or N- termini in other studies), and is more tunable to optimization (can tether cargoes other than ssODNs, i.e., small molecules and PEG to mitigate an immune response).
We envision that more conjugation methods will be developed across the field, and we anticipate that more studies will use these conjugation technologies to enhance CRISPR-based technologies. For example, we hypothesize that tethering NHEJ or p53 inhibitors to Cas9 could aid in the local inhibition of those proteins and greatly increase HDR while overcoming genotoxicity from global NHEJ inhibition or p53-induced cell death, respectively.
References:
- Hsu, P. D.; Lander, E. S.; Zhang, F., Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014,157(6), 1262-78.
- Barrangou, R.; Doudna, J. A., Applications of CRISPR technologies in research and beyond. Nat Biotechnol 2016,34(9), 933-941.
- Gangopadhyay, S. A.; Cox, K. J.; Manna, D.; Lim, D.; Maji, B.; Zhou, Q.; Choudhary, A., Precision Control of CRISPR-Cas9 Using Small Molecules and Light. Biochemistry 2019,58(4), 234-244.
- Lopez Del Amo, V.; Leger, B. S.; Cox, K. J.; Gill, S.; Bishop, A. L.; Scanlon, G. D.; Walker, J. A.; Gantz, V. M.; Choudhary, A., Small-Molecule Control of Super-Mendelian Inheritance in Gene Drives. Cell Rep 2020,31(13), 107841.
- Maji, B.; Moore, C. L.; Zetsche, B.; Volz, S. E.; Zhang, F.; Shoulders, M. D.; Choudhary, A., Multidimensional chemical control of CRISPR-Cas9. Nat Chem Biol 2017,13(1), 9-11.
- Manna, D.; Maji, B.; Gangopadhyay, S. A.; Cox, K. J.; Zhou, Q.; Law, B. K.; Mazitschek, R.; Choudhary, A., A Singular System with Precise Dosing and Spatiotemporal Control of CRISPR-Cas9. Angew Chem Int Ed Engl 2019,58(19), 6285-6289.
- Maji, B.; Gangopadhyay, S. A.; Lee, M.; Shi, M.; Wu, P.; Heler, R.; Mok, B.; Lim, D.; Siriwardena, S. U.; Paul, B.; Dancik, V.; Vetere, A.; Mesleh, M. F.; Marraffini, L. A.; Liu, D. R.; Clemons, P. A.; Wagner, B. K.; Choudhary, A., A High-Throughput Platform to Identify Small-Molecule Inhibitors of CRISPR-Cas9. Cell 2019,177(4), 1067-1079 e19.
- Lim, D.; Sreekanth, V.; Cox, K. J.; Law, B. K.; Wagner, B. K.; Karp, J. M.; Choudhary, A., Engineering designer beta cells with a CRISPR-Cas9 conjugation platform. Nature Communications 2020,11(1), 4043.
- Russell, M. A.; Morgan, N. G., The impact of anti-inflammatory cytokines on the pancreatic beta-cell. Islets 2014,6(3), e950547.
- Burns, S. M.; Vetere, A.; Walpita, D.; Dancik, V.; Khodier, C.; Perez, J.; Clemons, P. A.; Wagner, B. K.; Altshuler, D., High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab2015,21(1), 126-37.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in