A crosstalk between organelle and cytosolic environment
Published in Materials, Cell & Molecular Biology, and Education
For decades, lysosomes have been widely recognized as the cell’s highly acidic recycling centers, requiring a low internal pH to break down cellular waste. However, in our study, we reveals that the lysosomal surface is acidic as well-the luminal acidity spills over onto the outer surface, creating a nanointerface as a functional region.
To make this discovery, we developed ratiometric DNA nanodevices (DNs) equipped with pH-sensitive and pH-insensitive dyes. By stably anchoring these nanoscale tools specifically to the outer surface of living lysosomes, then we could precisely measure the microenvironment right outside the organelle. We uncovered a continuous, radiating acidic layer on the outside of all lysosomes that can reach up to 21 nm in thickness (Figure 1). This previously hidden nanolayer is typically 0.2 to 0.7 pH units more acidic than the surrounding neutral cytosolic pH.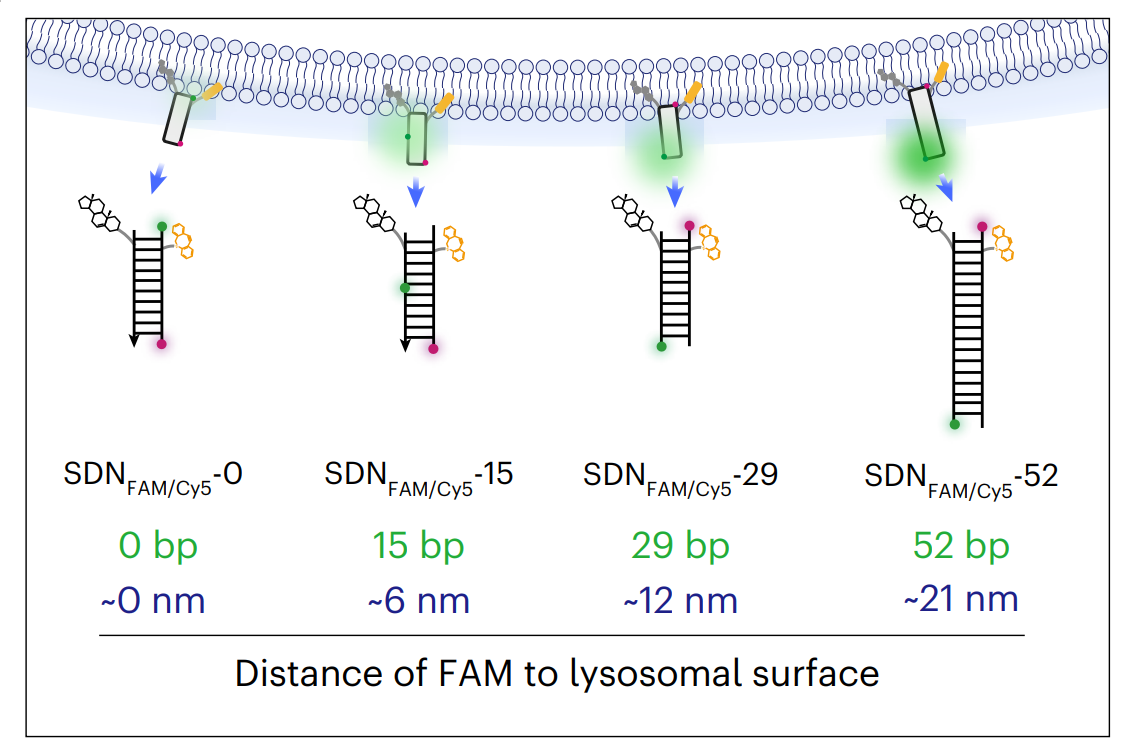
Figure 1. Schemetic illustration on DNA nanodevice-based thickness measurement.
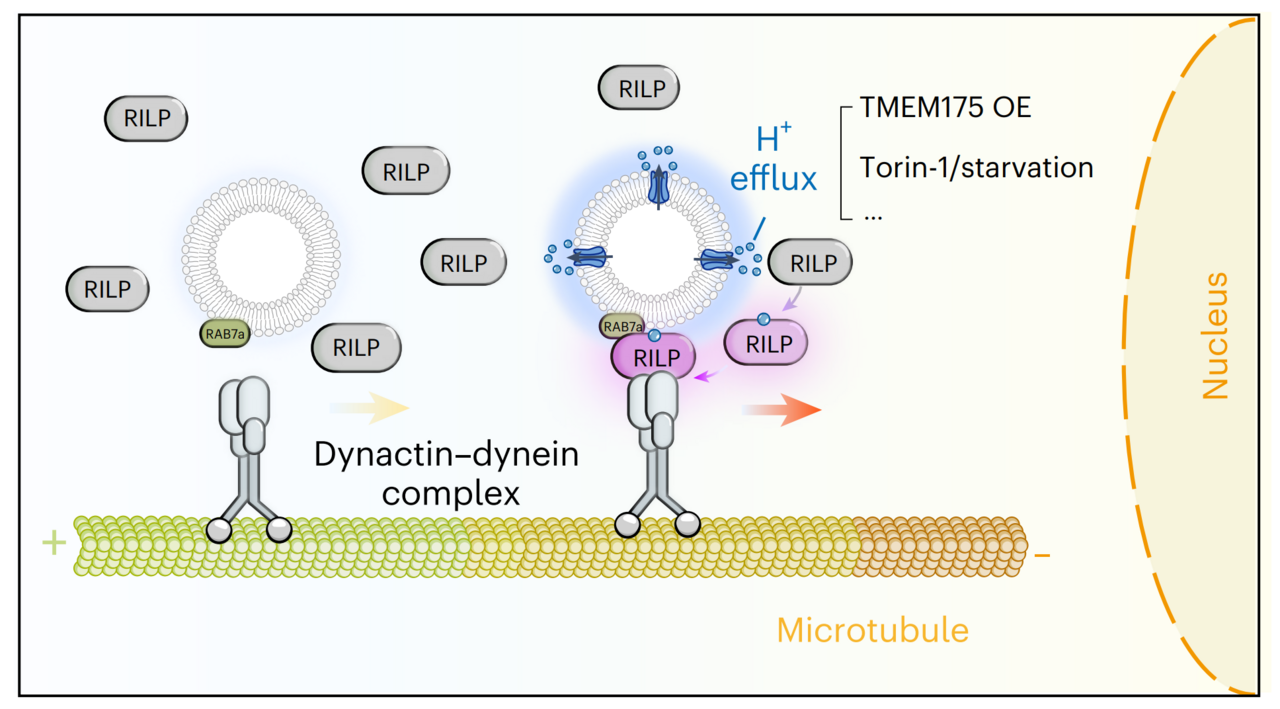
What powers this acidic nanolayer? We identified TMEM175,a known lysosomal proton channel associated with Parkinson’s disease as the primary engine (Figure 2). TMEM175 acts as a controlled "leak," allowing protons to flow out of the lysosome's lumen to establish and maintain this acidic nanolayer. When TMEM175 is disabled, this acidic nanolayer shrinks drastically.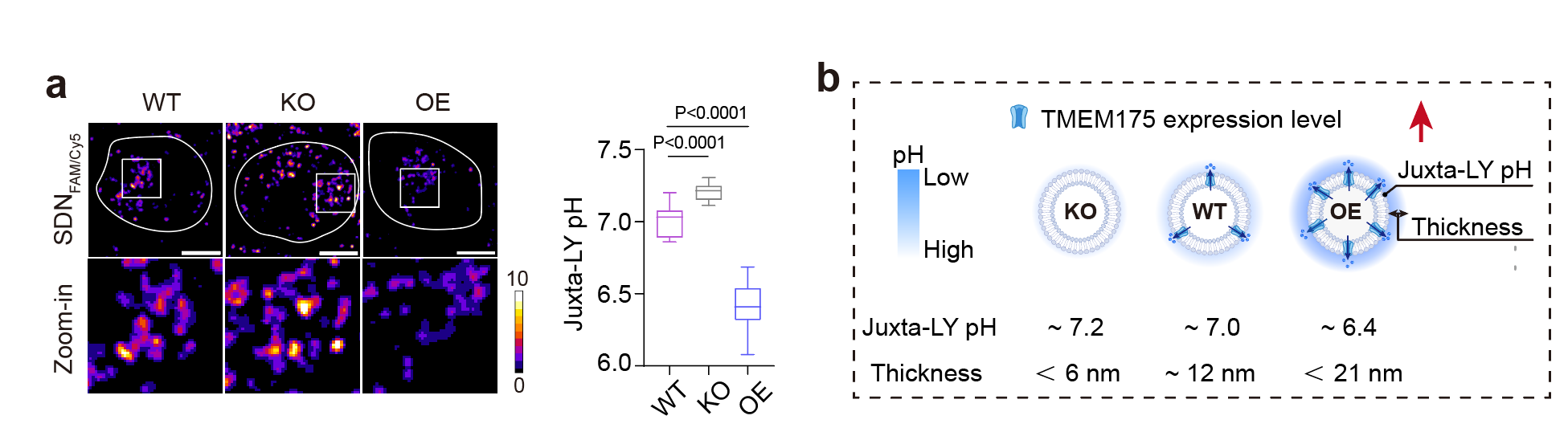
Figure 2. TMEM175 maintained the acidic nanolayer.(a) Wide type cells showed acidic juxta-lysosomal pH whearas TMEM175 KO cells and TMEM175 OE cells showed higher and lower juxta-lysosomal pH, respectively. (b) Summary figures on TMEM175-regulated lysosomal acidic nanolayer.
Crucially, this external acidity is not just a structural existence. Instead, it dictates how the organelle navigates in the cell. We found that the juxta-lysosomal pH, rather than the internal luminal pH, determines lysosome positioning. A cytosolic protein named RILP acts as a direct pH sensor for this acidic nanolayer. By sensing the acidity, RILP links the lysosome to motor proteins, driving the organelle toward the nucleus during nutrient starvation to perform its recycling duties.
Ultimately, this research proves that lysosomes utilize "inside-out" proton conduits to create a steady acidic nano-interface. This discovery fundamentally changes our understanding of how organelles communicate with the environment.
This work is highlighted in the News and Views of Nature Cell Biology and here is the link: https://www.nature.com/articles/s41556-025-01869-6
Follow the Topic
-
Nature Cell Biology

This journal publishes papers of the highest quality from all areas of cell biology, encouraging those that shed light on the mechanisms underlying fundamental cell biological processes.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Cell death and inflammatory signalling
Publishing Model: Hybrid
Deadline: Oct 28, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in